Isolation, N-glycosylations and Function of a Hyaluronidase-Like Enzyme from the Venom of the Spider Cupiennius salei
- PMID: 26630650
- PMCID: PMC4667920
- DOI: 10.1371/journal.pone.0143963
Isolation, N-glycosylations and Function of a Hyaluronidase-Like Enzyme from the Venom of the Spider Cupiennius salei
Abstract
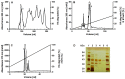
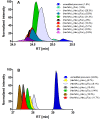
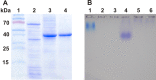
Structure of cupiennius salei venom hyaluronidase: Hyaluronidases are important venom components acting as spreading factor of toxic compounds. In several studies this spreading effect was tested on vertebrate tissue. However, data about the spreading activity on invertebrates, the main prey organisms of spiders, are lacking. Here, a hyaluronidase-like enzyme was isolated from the venom of the spider Cupiennius salei. The amino acid sequence of the enzyme was determined by cDNA analysis of the venom gland transcriptome and confirmed by protein analysis. Two complex N-linked glycans akin to honey bee hyaluronidase glycosylations, were identified by tandem mass spectrometry. A C-terminal EGF-like domain was identified in spider hyaluronidase using InterPro. The spider hyaluronidase-like enzyme showed maximal activity at acidic pH, between 40-60°C, and 0.2 M KCl. Divalent ions did not enhance HA degradation activity, indicating that they are not recruited for catalysis.
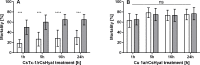
Function of venom hyaluronidases: Besides hyaluronan, the enzyme degrades chondroitin sulfate A, whereas heparan sulfate and dermatan sulfate are not affected. The end products of hyaluronan degradation are tetramers, whereas chondroitin sulfate A is mainly degraded to hexamers. Identification of terminal N-acetylglucosamine or N-acetylgalactosamine at the reducing end of the oligomers identified the enzyme as an endo-β-N-acetyl-D-hexosaminidase hydrolase. The spreading effect of the hyaluronidase-like enzyme on invertebrate tissue was studied by coinjection of the enzyme with the Cupiennius salei main neurotoxin CsTx-1 into Drosophila flies. The enzyme significantly enhances the neurotoxic activity of CsTx-1. Comparative substrate degradation tests with hyaluronan, chondroitin sulfate A, dermatan sulfate, and heparan sulfate with venoms from 39 spider species from 21 families identified some spider families (Atypidae, Eresidae, Araneidae and Nephilidae) without activity of hyaluronidase-like enzymes. This is interpreted as a loss of this enzyme and fits quite well the current phylogenetic idea on a more isolated position of these families and can perhaps be explained by specialized prey catching techniques.
Conflict of interest statement
Figures








Similar articles
-
Characterization of hyaluronidase isolated from Agkistrodon contortrix contortrix (Southern Copperhead) venom.Arch Biochem Biophys. 2001 Feb 15;386(2):154-62. doi: 10.1006/abbi.2000.2204. Arch Biochem Biophys. 2001. PMID: 11368337
-
Biochemistry, toxicology and ecology of the venom of the spider Cupiennius salei (Ctenidae).Toxicon. 2004 Apr;43(5):543-53. doi: 10.1016/j.toxicon.2004.02.009. Toxicon. 2004. PMID: 15066412 Review.
-
Hyaluronidases in Loxosceles intermedia (Brown spider) venom are endo-beta-N-acetyl-d-hexosaminidases hydrolases.Toxicon. 2007 May;49(6):758-68. doi: 10.1016/j.toxicon.2006.11.024. Epub 2006 Dec 2. Toxicon. 2007. PMID: 17210169
-
Purification and properties of hyaluronidase from Hippasa partita (funnel web spider) venom gland extract.Toxicon. 2007 Sep 1;50(3):383-93. doi: 10.1016/j.toxicon.2007.04.007. Epub 2007 Apr 24. Toxicon. 2007. PMID: 17548101
-
Recent advances in the understanding of brown spider venoms: From the biology of spiders to the molecular mechanisms of toxins.Toxicon. 2014 Jun;83:91-120. doi: 10.1016/j.toxicon.2014.02.023. Epub 2014 Mar 11. Toxicon. 2014. PMID: 24631373 Review.
Cited by
-
Origin and Characterization of Extracellular Vesicles Present in the Spider Venom of Ornithoctonus hainana.Toxins (Basel). 2021 Aug 20;13(8):579. doi: 10.3390/toxins13080579. Toxins (Basel). 2021. PMID: 34437450 Free PMC article.
-
Proteomic analysis of the venom of Conus flavidus from Red Sea reveals potential pharmacological applications.J Genet Eng Biotechnol. 2024 Jun;22(2):100375. doi: 10.1016/j.jgeb.2024.100375. Epub 2024 Apr 27. J Genet Eng Biotechnol. 2024. PMID: 38797555 Free PMC article.
-
Characterisation of protein families in spider digestive fluids and their role in extra-oral digestion.BMC Genomics. 2017 Aug 10;18(1):600. doi: 10.1186/s12864-017-3987-9. BMC Genomics. 2017. PMID: 28797246 Free PMC article.
-
Identification of a precursor processing protease from the spider Cupiennius salei essential for venom neurotoxin maturation.J Biol Chem. 2018 Feb 9;293(6):2079-2090. doi: 10.1074/jbc.M117.810911. Epub 2017 Dec 21. J Biol Chem. 2018. PMID: 29269415 Free PMC article.
-
Overview of protein posttranslational modifications in Arthropoda venoms.J Venom Anim Toxins Incl Trop Dis. 2022 Apr 15;28:e20210047. doi: 10.1590/1678-9199-JVATITD-2021-0047. eCollection 2022. J Venom Anim Toxins Incl Trop Dis. 2022. PMID: 35519418 Free PMC article. Review.
References
-
- Stern R, Kogan G, Jedrzejas MJ, Šoltés L. The many ways to cleave hyaluronan. Biotechnol Adv. 2007;25(6):537–557. - PubMed
-
- Sugahara K, Mikami T, Uyama T, Mizuguchi S, Nomura K, Kitagawa H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol. 2003;13(5):612–620. - PubMed
-
- Feng L, Gao R, Gopalakrishnakone P. Isolation and characterization of a hyaluronidase from the venom of Chinese red scorpion Buthus martensi . Comp Biochem Physiol Toxicol Pharmacol. 2008;148(3):250–257. - PubMed
-
- Kemeny DM, Dalton N, Lawrence AJ, Pearce FL, Vernon CA. The purification and characterisation of hyaluronidase from the venom of the honey bee, Apis mellifera . Eur J Biochem. 1984;139(2):217–223. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

