Transfection with thymidine kinase permits bromodeoxyuridine labelling of DNA replication in the human malaria parasite Plasmodium falciparum
- PMID: 26630917
- PMCID: PMC4668656
- DOI: 10.1186/s12936-015-1014-7
Transfection with thymidine kinase permits bromodeoxyuridine labelling of DNA replication in the human malaria parasite Plasmodium falciparum
Abstract
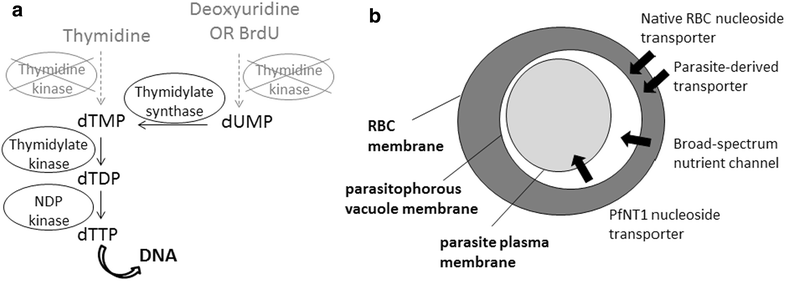
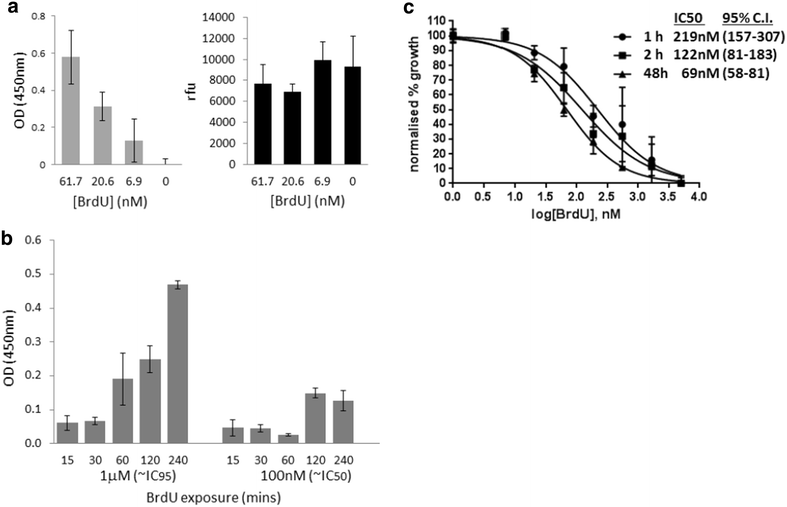
Background: Plasmodium falciparum, the causative agent of severe human malaria, is an early-diverging protozoan whose lifecycle has many unusual features, including its modes of replication. Research on the Plasmodium cell cycle, which occurs primarily via schizogony instead of canonical binary fission, has been hampered by a lack of tools and markers that can be transferred from cell cycle studies in model organisms. A common tool used to study DNA replication and the cell cycle in human cells is the labelling of newly-replicated DNA with the modified nucleotide bromodeoxyuridine (BrdU), followed by immunofluorescent detection. Plasmodium parasites, however, do not incorporate BrdU because they rely only on de novo synthesis of pyrimidines and do not salvage thymidine analogues like BrdU for conversion into nucleotides.
Methods: Analysis of biochemical pathways in Plasmodium indicated that the absence of the enzyme thymidine kinase (TK) may be the only impediment to BrdU incorporation in this organism. A TK gene from Herpes simplex was, therefore, introduced into the Plasmodium falciparum 3D7 strain and the effect on BrdU labelling was assessed by enzyme-linked immunosorbent assay and immunofluorescence microscopy.
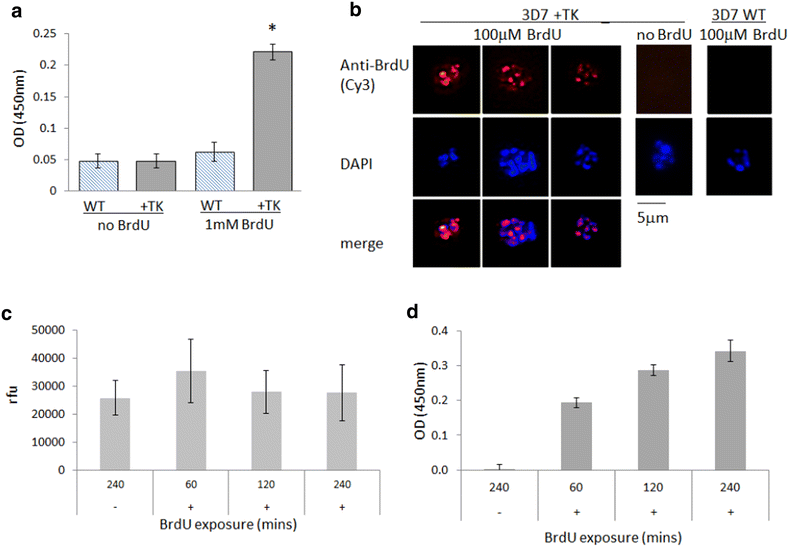
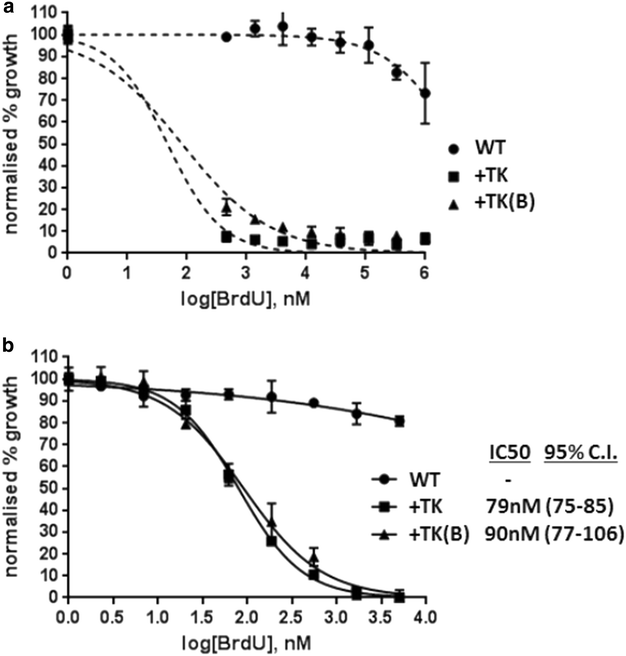
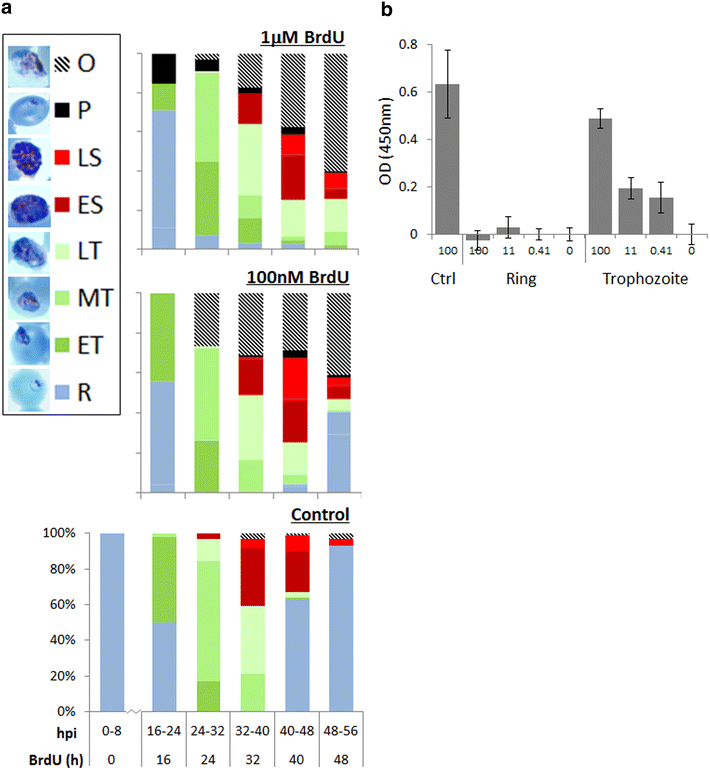
Results: Introduction of a TK gene produces parasites that can indeed incorporate BrdU. This forms a sensitive indicator of DNA replication, which can be detected by both quantitative and qualitative assays on either a population level or a single-cell level. Plasmodium falciparum, when expressing TK, becomes unusually sensitive to BrdU toxicity.
Conclusions: BrdU labelling represents a significant new tool for investigating DNA replication and the cell cycle in Plasmodium.
Figures
References
-
- WHO: World Malaria Report 2014. Geneva, World Health Organization, 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

