Middle East respiratory syndrome coronavirus ORF4b protein inhibits type I interferon production through both cytoplasmic and nuclear targets
- PMID: 26631542
- PMCID: PMC4668369
- DOI: 10.1038/srep17554
Middle East respiratory syndrome coronavirus ORF4b protein inhibits type I interferon production through both cytoplasmic and nuclear targets
Abstract
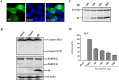
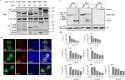
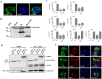
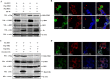
Middle East respiratory syndrome coronavirus (MERS-CoV) is a novel and highly pathogenic human coronavirus and has quickly spread to other countries in the Middle East, Europe, North Africa and Asia since 2012. Previous studies have shown that MERS-CoV ORF4b antagonizes the early antiviral alpha/beta interferon (IFN-α/β) response, which may significantly contribute to MERS-CoV pathogenesis; however, the underlying mechanism is poorly understood. Here, we found that ORF4b in the cytoplasm could specifically bind to TANK binding kinase 1 (TBK1) and IκB kinase epsilon (IKKε), suppress the molecular interaction between mitochondrial antiviral signaling protein (MAVS) and IKKε, and inhibit IFN regulatory factor 3 (IRF3) phosphorylation and subsequent IFN-β production. Further analysis showed that ORF4b could also inhibit IRF3 and IRF7-induced production of IFN-β, whereas deletion of the nuclear localization signal of ORF4b abrogated its ability to inhibit IRF3 and IRF7-induced production of IFN-β, but not IFN-β production induced by RIG-I, MDA5, MAVS, IKKε, and TBK-1, suggesting that ORF4b could inhibit the induction of IFN-β in both the cytoplasm and nucleus. Collectively, these results indicate that MERS-CoV ORF4b inhibits the induction of type I IFN through a direct interaction with IKKε/TBK1 in the cytoplasm, and also in the nucleus with unknown mechanism. Viruses have evolved multiple strategies to evade or thwart a host's antiviral responses. A novel human coronavirus (HCoV), Middle East respiratory syndrome coronavirus (MERS-CoV), is distinguished from other coronaviruses by its high pathogenicity and mortality. However, virulence determinants that distinguish MERS-CoV from other HCoVs have yet to be identified. MERS-CoV ORF4b antagonizes the early antiviral response, which may contribute to MERS-CoV pathogenesis. Here, we report the identification of the interferon (IFN) antagonism mechanism of MERS-CoV ORF4b. MERS-CoV ORF4b inhibits the production of type I IFN through a direct interaction with IKKε/TBK1 in the cytoplasm, and also in the nucleus with unknown mechanism. These findings provide a rationale for the novel pathogenesis of MERS-CoV as well as a basis for developing a candidate therapeutic against this virus.
Figures


Similar articles
-
Middle East respiratory syndrome coronavirus-encoded ORF8b strongly antagonizes IFN-β promoter activation: its implication for vaccine design.J Microbiol. 2019 Sep;57(9):803-811. doi: 10.1007/s12275-019-9272-7. Epub 2019 Aug 27. J Microbiol. 2019. PMID: 31452044 Free PMC article.
-
MERS-CoV-nsp5 expression in human epithelial BEAS 2b cells attenuates type I interferon production by inhibiting IRF3 nuclear translocation.Cell Mol Life Sci. 2024 Oct 12;81(1):433. doi: 10.1007/s00018-024-05458-y. Cell Mol Life Sci. 2024. PMID: 39395053 Free PMC article.
-
Middle East respiratory syndrome coronavirus M protein suppresses type I interferon expression through the inhibition of TBK1-dependent phosphorylation of IRF3.Emerg Microbes Infect. 2016 Apr 20;5(4):e39. doi: 10.1038/emi.2016.33. Emerg Microbes Infect. 2016. PMID: 27094905 Free PMC article.
-
Modulation of the immune response by Middle East respiratory syndrome coronavirus.J Cell Physiol. 2019 Mar;234(3):2143-2151. doi: 10.1002/jcp.27155. Epub 2018 Aug 26. J Cell Physiol. 2019. PMID: 30146782 Free PMC article. Review.
-
[Role of IPS-1 in type I IFN induction].Nihon Rinsho. 2006 Jul;64(7):1231-5. Nihon Rinsho. 2006. PMID: 16841392 Review. Japanese.
Cited by
-
Potential Antiviral Immune Response Against COVID-19: Lessons Learned from SARS-CoV.Adv Exp Med Biol. 2021;1318:149-167. doi: 10.1007/978-3-030-63761-3_9. Adv Exp Med Biol. 2021. PMID: 33973177
-
UBR5 Acts as an Antiviral Host Factor against MERS-CoV via Promoting Ubiquitination and Degradation of ORF4b.J Virol. 2022 Sep 14;96(17):e0074122. doi: 10.1128/jvi.00741-22. Epub 2022 Aug 18. J Virol. 2022. PMID: 35980206 Free PMC article.
-
Transgene expression in the genome of Middle East respiratory syndrome coronavirus based on a novel reverse genetics system utilizing Red-mediated recombination cloning.J Gen Virol. 2017 Oct;98(10):2461-2469. doi: 10.1099/jgv.0.000919. J Gen Virol. 2017. PMID: 28984231 Free PMC article.
-
MERS coronaviruses from camels in Africa exhibit region-dependent genetic diversity.Proc Natl Acad Sci U S A. 2018 Mar 20;115(12):3144-3149. doi: 10.1073/pnas.1718769115. Epub 2018 Mar 5. Proc Natl Acad Sci U S A. 2018. PMID: 29507189 Free PMC article.
-
Cytokine storm in aged people with CoV-2: possible role of vitamins as therapy or preventive strategy.Aging Clin Exp Res. 2020 Oct;32(10):2115-2131. doi: 10.1007/s40520-020-01669-y. Epub 2020 Aug 31. Aging Clin Exp Res. 2020. PMID: 32865757 Free PMC article. Review.
References
-
- Zaki A. M., van Boheemen S., Bestebroer T. M., Osterhaus A. D. & Fouchier R. A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. The New England journal of medicine 367, 1814–1820 (2012). - PubMed
-
- World Health Organization. Novel coronavirus infection - update. (2015), http://www.who.int/csr/don/12-october-2015-mers-saudi-arabia/en/. [Accessed October 12, 2015].
-
- Butler D. Clusters of coronavirus cases put scientists on alert. Nature 492, 166–167 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

