A role of the sphingosine-1-phosphate (S1P)-S1P receptor 2 pathway in epithelial defense against cancer (EDAC)
- PMID: 26631556
- PMCID: PMC4751600
- DOI: 10.1091/mbc.E15-03-0161
A role of the sphingosine-1-phosphate (S1P)-S1P receptor 2 pathway in epithelial defense against cancer (EDAC)
Abstract
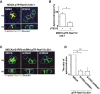
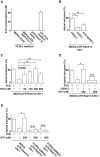
At the initial step of carcinogenesis, transformation occurs in single cells within epithelia, where the newly emerging transformed cells are surrounded by normal epithelial cells. A recent study revealed that normal epithelial cells have an ability to sense and actively eliminate the neighboring transformed cells, a process named epithelial defense against cancer (EDAC). However, the molecular mechanism of this tumor-suppressive activity is largely unknown. In this study, we investigated a role for the sphingosine-1-phosphate (S1P)-S1P receptor 2 (S1PR2) pathway in EDAC. First, we show that addition of the S1PR2 inhibitor significantly suppresses apical extrusion of RasV12-transformed cells that are surrounded by normal cells. In addition, knockdown of S1PR2 in normal cells induces the same effect, indicating that S1PR2 in the surrounding normal cells plays a positive role in the apical elimination of the transformed cells. Of importance, not endogenous S1P but exogenous S1P is involved in this process. By using FRET analyses, we demonstrate that S1PR2 mediates Rho activation in normal cells neighboring RasV12-transformed cells, thereby promoting accumulation of filamin, a crucial regulator of EDAC. Collectively these data indicate that S1P is a key extrinsic factor that affects the outcome of cell competition between normal and transformed epithelial cells.
© 2016 Yamamoto, Yako, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



References
-
- Edsall LC, Spiegel S. Enzymatic measurement of sphingosine 1-phosphate. Anal Biochem. 1999;272:80–86. - PubMed
-
- Fujioka Y, Tsuda M, Nanbo A, Hattori T, Sasaki J, Sasaki T, Miyazaki T, Ohba Y. A Ca(2+)-dependent signalling circuit regulates influenza A virus internalization and infection. Nat Commun. 2013;4:2763. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

