Experimental models of hepatotoxicity related to acute liver failure
- PMID: 26631581
- PMCID: PMC4691574
- DOI: 10.1016/j.taap.2015.11.016
Experimental models of hepatotoxicity related to acute liver failure
Abstract
Acute liver failure can be the consequence of various etiologies, with most cases arising from drug-induced hepatotoxicity in Western countries. Despite advances in this field, the management of acute liver failure continues to be one of the most challenging problems in clinical medicine. The availability of adequate experimental models is of crucial importance to provide a better understanding of this condition and to allow identification of novel drug targets, testing the efficacy of new therapeutic interventions and acting as models for assessing mechanisms of toxicity. Experimental models of hepatotoxicity related to acute liver failure rely on surgical procedures, chemical exposure or viral infection. Each of these models has a number of strengths and weaknesses. This paper specifically reviews commonly used chemical in vivo and in vitro models of hepatotoxicity associated with acute liver failure.
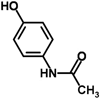
Keywords: Acetaminophen; Acute liver failure; Concanavalin A; D-galactosamine; Fas ligand; Hepatotoxicity.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Abe K, Ohira H, Kobayashi H, Rai T, Saito H, Takahashi A, Sato Y. Role of CpG ODN in concanavalin A-induced hepatitis in mice. Fukushima J Med Sci. 2005;51:41–49. - PubMed
-
- Abou-Elella AM, Siendones E, Padillo J, Montero JL, De la Mata M, Muntane Relat J. Tumour necrosis factor-alpha and nitric oxide mediate apoptosis by D-galactosamine in a primary culture of rat hepatocytes: exacerbation of cell death by cocultured Kupffer cells. Can J Gastroenterol. 2002;16:791–799. - PubMed
-
- Adamson GM, Harman AW. Oxidative stress in cultured hepatocytes exposed to acetaminophen. Biochem Pharmacol. 1993;45:2289–2294. - PubMed
-
- Albano E, Rundgren M, Harvison PJ, Nelson SD, Moldéus P. Mechanisms of N-acetyl-p-benzoquinone imine cytotoxicity. Mol Pharmacol. 1985;28:306–311. - PubMed
-
- Aninat C, et al. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006;34:75–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

