TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma
- PMID: 26631690
- PMCID: PMC4668567
- DOI: 10.1038/srep17622
TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma
Abstract
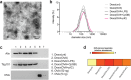
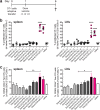
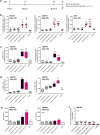
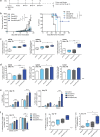
Dendritic cell (DC)-derived exosomes (Dexo) contain the machinery necessary to activate potent antigen-specific immune responses. As promising cell-free immunogens, Dexo have been tested in previous clinical trials for cancer vaccine immunotherapy, yet resulted in limited therapeutic benefit. Here, we explore a novel Dexo vaccine formulation composed of Dexo purified from DCs loaded with antigens and matured with either the TLR-3 ligand poly(I:C), the TLR-4 ligand LPS or the TLR-9 ligand CpG-B. When poly(I:C) was used to produce exosomes together with ovalbumin (OVA), the resulting Dexo vaccine strongly stimulated OVA-specific CD8(+) and CD4(+) T cells to proliferate and acquire effector functions. When a B16F10 melanoma cell lysate was used to load DCs with tumor antigens during exosome production together with poly(I:C), we obtained a Dexo vaccine capable of inducing robust activation of melanoma-specific CD8(+) T cells and the recruitment of cytotoxic CD8(+) T cells, NK and NK-T cells to the tumor site, resulting in significantly reduced tumor growth and enhanced survival as compared to a Dexo vaccine formulation similar to the one previously tested on human patients. Our results indicate that poly(I:C) is a particularly favorable TLR agonist for DC maturation during antigen loading and exosome production for cancer immunotherapy.
Figures


References
-
- Chaput N. et al. Dendritic cell derived-exosomes: biology and clinical implementations. J. Leukoc. Biol. 80, 471–478 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

