The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration
- PMID: 26631828
- PMCID: PMC4924535
- DOI: 10.1016/j.jcyt.2015.10.008
The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration
Abstract
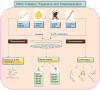
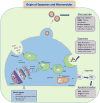
The unique properties of mesenchymal stromal/stem cells (MSCs) to self-renew and their multipotentiality have rendered them attractive to researchers and clinicians. In addition to the differentiation potential, the broad repertoire of secreted trophic factors (cytokines) exhibiting diverse functions such as immunomodulation, anti-inflammatory activity, angiogenesis and anti-apoptotic, commonly referred to as the MSC secretome, has gained immense attention in the past few years. There is enough evidence to show that the one important pathway by which MSCs participate in tissue repair and regeneration is through its secretome. Concurrently, a large body of MSC research has focused on characterization of the MSC secretome; this includes both soluble factors and factors released in extracellular vesicles, for example, exosomes and microvesicles. This review provides an overview of our current understanding of the MSC secretome with respect to their potential clinical applications.
Keywords: cell therapy; exosomes; mesenchymal stromal cells; regeneration; secretome.
Copyright © 2015 International Society for Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. - PubMed
-
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–62. - PubMed
-
- Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20(5):510–18. - PubMed
-
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

