Mica Nanoparticle, STB-HO Eliminates the Human Breast Carcinoma Cells by Regulating the Interaction of Tumor with its Immune Microenvironment
- PMID: 26631982
- PMCID: PMC4668362
- DOI: 10.1038/srep17515
Mica Nanoparticle, STB-HO Eliminates the Human Breast Carcinoma Cells by Regulating the Interaction of Tumor with its Immune Microenvironment
Abstract
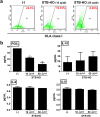
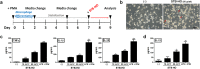
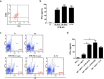
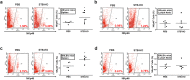
Mica, an aluminosilicate mineral, has been proven to possess anti-tumor and immunostimulatory effects. However, its efficacy and mechanisms in treating various types of tumor are less verified and the mechanistic link between anti-tumor and immunostimulatory effects has not been elucidated. We sought to investigate the therapeutic effect of STB-HO (mica nanoparticles) against one of the most prevalent cancers, the breast cancer. STB-HO was orally administered into MCF-7 xenograft model or directly added to culture media and tumor growth was monitored. STB-HO administration exhibited significant suppressive effects on the growth of MCF-7 cells in vivo, whereas STB-HO did not affect the proliferation and apoptosis of MCF-7 cells in vitro. To address this discrepancy between in vivo and in vitro results, we investigated the effects of STB-HO treatment on the interaction of MCF-7 cells with macrophages, dendritic cells (DCs) and natural killer (NK) cells, which constitute the cellular composition of tumor microenvironment. Importantly, STB-HO not only increased the susceptibility of MCF-7 cells to immune cells, but also stimulated the immunocytes to eliminate cancer cells. In conclusion, our study highlights the possible role of STB-HO in the suppression of MCF-7 cell growth via the regulation of interactions between tumor cells and anti-tumor immune cells.
Figures




Similar articles
-
Particled Mica, STB-HO has chemopreventive potential via G1 arrest, and inhibition of proliferation and vascular endothelial growth factor receptor 2 in HCT colorectal cancer cells.BMC Complement Altern Med. 2013 Jul 24;13:189. doi: 10.1186/1472-6882-13-189. BMC Complement Altern Med. 2013. PMID: 23883349 Free PMC article.
-
STB-HO, a novel mica fine particle, inhibits the teratoma-forming ability of human embryonic stem cells after in vivo transplantation.Oncotarget. 2016 Jan 19;7(3):2684-95. doi: 10.18632/oncotarget.6472. Oncotarget. 2016. PMID: 26646796 Free PMC article.
-
The antitumor efficacy of functional paclitaxel nanomicelles in treating resistant breast cancers by oral delivery.Biomaterials. 2011 Apr;32(12):3285-302. doi: 10.1016/j.biomaterials.2011.01.038. Biomaterials. 2011. PMID: 21306774
-
Effect of tumor cells and tumor microenvironment on NK-cell function.Eur J Immunol. 2014 Jun;44(6):1582-92. doi: 10.1002/eji.201344272. Eur J Immunol. 2014. PMID: 24777896 Review.
-
The Tumor Microenvironment of Primitive and Metastatic Breast Cancer: Implications for Novel Therapeutic Strategies.Int J Mol Sci. 2020 Oct 30;21(21):8102. doi: 10.3390/ijms21218102. Int J Mol Sci. 2020. PMID: 33143050 Free PMC article. Review.
Cited by
-
Nanotechnology-Aided Advancement in Combating the Cancer Metastasis.Pharmaceuticals (Basel). 2023 Jun 19;16(6):899. doi: 10.3390/ph16060899. Pharmaceuticals (Basel). 2023. PMID: 37375846 Free PMC article. Review.
-
A Nanorobotics-Based Approach of Breast Cancer in the Nanotechnology Era.Int J Mol Sci. 2024 May 2;25(9):4981. doi: 10.3390/ijms25094981. Int J Mol Sci. 2024. PMID: 38732200 Free PMC article. Review.
-
Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis.Cell Death Dis. 2016 Dec 22;7(12):e2524. doi: 10.1038/cddis.2016.442. Cell Death Dis. 2016. PMID: 28005072 Free PMC article.
-
The Effect of a Novel Mica Nanoparticle, STB-MP, on an Alzheimer's Disease Patient-Induced PSC-Derived Cortical Brain Organoid Model.Nanomaterials (Basel). 2023 Feb 27;13(5):893. doi: 10.3390/nano13050893. Nanomaterials (Basel). 2023. PMID: 36903771 Free PMC article.
-
MIS416 Enhances Therapeutic Functions of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Against Experimental Colitis by Modulating Systemic Immune Milieu.Front Immunol. 2018 May 28;9:1078. doi: 10.3389/fimmu.2018.01078. eCollection 2018. Front Immunol. 2018. PMID: 29892282 Free PMC article.
References
-
- Blagosklonny M. V. Matching targets for selective cancer therapy. Drug Discov Today 8, 1104–1107 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

