Recombinant Resilin-Based Bioelastomers for Regenerative Medicine Applications
- PMID: 26632334
- PMCID: PMC4754112
- DOI: 10.1002/adhm.201500411
Recombinant Resilin-Based Bioelastomers for Regenerative Medicine Applications
Abstract
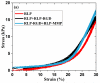
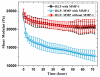
The outstanding elasticity, excellent resilience at high-frequency, and hydrophilic capacity of natural resilin have motivated investigations of recombinant resilin-based biomaterials as a new class of bio-elastomers in the engineering of mechanically active tissues. Accordingly, here the comprehensive characterization of modular resilin-like polypeptide (RLP) hydrogels is presented and their suitability as a novel biomaterial for in vivo applications is introduced. Oscillatory rheology confirmed that a full suite of the RLPs can be rapidly cross-linked upon addition of the tris(hydroxymethyl phosphine) cross-linker, achieving similar in situ shear storage moduli (20 k ± 3.5 Pa) across various material compositions. Uniaxial stress relaxation tensile testing of hydrated RLP hydrogels under cyclic loading and unloading showed negligible stress reduction and hysteresis, superior reversible extensibility, and high resilience with Young's moduli of 30 ± 7.4 kPa. RLP hydrogels containing MMP-sensitive domains are susceptible to enzymatic degradation by matrix metalloproteinase-1 (MMP-1). Cell culture studies revealed that RLP-based hydrogels supported the attachment and spreading (2D) of human mesenchymal stem cells and did not activate cultured macrophages. Subcutaneous transplantation of RLP hydrogels in a rat model, which to our knowledge is the first such reported in vivo analysis of RLP-based hydrogels, illustrated that these materials do not elicit a significant inflammatory response, suggesting their potential as materials for tissue engineering applications with targets of mechanically demanding tissues such as vocal fold and cardiovascular tissues.
Keywords: bio-elastomer; hydrogel; resilin-like polypeptide; tissue engineering; vocal fold.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures










Similar articles
-
Transient dynamic mechanical properties of resilin-based elastomeric hydrogels.Front Chem. 2014 Apr 28;2:21. doi: 10.3389/fchem.2014.00021. eCollection 2014. Front Chem. 2014. PMID: 24809044 Free PMC article.
-
Resilin-PEG Hybrid Hydrogels Yield Degradable Elastomeric Scaffolds with Heterogeneous Microstructure.Biomacromolecules. 2016 Jan 11;17(1):128-40. doi: 10.1021/acs.biomac.5b01255. Epub 2015 Dec 22. Biomacromolecules. 2016. PMID: 26646060 Free PMC article.
-
Tunable mechanical stability and deformation response of a resilin-based elastomer.Biomacromolecules. 2011 Jun 13;12(6):2302-10. doi: 10.1021/bm200373p. Epub 2011 May 25. Biomacromolecules. 2011. PMID: 21553895 Free PMC article.
-
Resilin-mimetics as a smart biomaterial platform for biomedical applications.Nat Commun. 2021 Jan 8;12(1):149. doi: 10.1038/s41467-020-20375-x. Nat Commun. 2021. PMID: 33420053 Free PMC article. Review.
-
Resilin: protein-based elastomeric biomaterials.Acta Biomater. 2014 Apr;10(4):1601-11. doi: 10.1016/j.actbio.2013.06.038. Epub 2013 Jul 3. Acta Biomater. 2014. PMID: 23831198 Review.
Cited by
-
Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration.Biomaterials. 2016 Nov;108:91-110. doi: 10.1016/j.biomaterials.2016.08.054. Epub 2016 Sep 2. Biomaterials. 2016. PMID: 27619243 Free PMC article. Review.
-
An in vitro assessment of the response of THP-1 macrophages to varying stiffness of a glycol-chitosan hydrogel for vocal fold tissue engineering applications.J Biomed Mater Res A. 2021 Aug;109(8):1337-1352. doi: 10.1002/jbm.a.37125. Epub 2020 Nov 6. J Biomed Mater Res A. 2021. PMID: 33112473 Free PMC article.
-
Characterization of resilin-like proteins with tunable mechanical properties.J Mech Behav Biomed Mater. 2019 Mar;91:68-75. doi: 10.1016/j.jmbbm.2018.11.015. Epub 2018 Nov 20. J Mech Behav Biomed Mater. 2019. PMID: 30544024 Free PMC article.
-
Sequence-Encoded Differences in Phase Separation Enable Formation of Resilin-like Polypeptide-Based Microstructured Hydrogels.Biomacromolecules. 2023 Aug 14;24(8):3729-3741. doi: 10.1021/acs.biomac.3c00418. Epub 2023 Jul 31. Biomacromolecules. 2023. PMID: 37525441 Free PMC article.
-
Modifying Naturally Occurring, Nonmammalian-Sourced Biopolymers for Biomedical Applications.ACS Biomater Sci Eng. 2024 Oct 14;10(10):5915-5938. doi: 10.1021/acsbiomaterials.4c00689. Epub 2024 Sep 11. ACS Biomater Sci Eng. 2024. PMID: 39259773 Review.
References
-
- Olsen BD. AIChE J. 2013;59:3558.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

