Human Germline CRISPR-Cas Modification: Toward a Regulatory Framework
- PMID: 26632357
- PMCID: PMC4699477
- DOI: 10.1080/15265161.2015.1104160
Human Germline CRISPR-Cas Modification: Toward a Regulatory Framework
Abstract
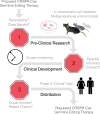
CRISPR germline editing therapies (CGETs) hold unprecedented potential to eradicate hereditary disorders. However, the prospect of altering the human germline has sparked a debate over the safety, efficacy, and morality of CGETs, triggering a funding moratorium by the NIH. There is an urgent need for practical paths for the evaluation of these capabilities. We propose a model regulatory framework for CGET research, clinical development, and distribution. Our model takes advantage of existing legal and regulatory institutions but adds elevated scrutiny at each stage of CGET development to accommodate the unique technical and ethical challenges posed by germline editing.
Keywords: CRISPR-Cas; clinical ethics; gene editing; gene therapy; genetic disease; germline modification.
Figures
Comment in
-
Response to Open Peer Commentaries on "Human Germline CRISPR-Cas Modification: Toward a Regulatory Framework".Am J Bioeth. 2016 Oct;16(10):W1-2. doi: 10.1080/15265161.2016.1214308. Am J Bioeth. 2016. PMID: 27653416 No abstract available.
Similar articles
-
GENETIC ENGINEERING. Germline editing dominates DNA summit.Science. 2015 Dec 11;350(6266):1299-300. doi: 10.1126/science.350.6266.1299. Science. 2015. PMID: 26659031 No abstract available.
-
Germline Manipulation and Our Future Worlds.Am J Bioeth. 2015;15(12):30-4. doi: 10.1080/15265161.2015.1104163. Am J Bioeth. 2015. PMID: 26632358
-
CRISPR Critters and CRISPR Cracks.Am J Bioeth. 2015;15(12):11-7. doi: 10.1080/15265161.2015.1104138. Am J Bioeth. 2015. PMID: 26632355
-
International regulatory landscape and integration of corrective genome editing into in vitro fertilization.Reprod Biol Endocrinol. 2014 Nov 24;12:108. doi: 10.1186/1477-7827-12-108. Reprod Biol Endocrinol. 2014. PMID: 25420886 Free PMC article. Review.
-
The case for germline gene correction: state of the science.Fertil Steril. 2025 Jul;124(1):22-29. doi: 10.1016/j.fertnstert.2025.04.039. Epub 2025 May 5. Fertil Steril. 2025. PMID: 40334730 Review.
Cited by
-
Initial heritable genome editing: mapping a responsible pathway from basic research to the clinic.Med Health Care Philos. 2023 Mar;26(1):21-35. doi: 10.1007/s11019-022-10115-x. Epub 2022 Nov 22. Med Health Care Philos. 2023. PMID: 36414813 Free PMC article.
-
Safety of Germline Genome Editing for Genetically Related "Future" Children as Perceived by Parents.CRISPR J. 2019 Dec;2(6):370-375. doi: 10.1089/crispr.2019.0010. Epub 2019 Nov 19. CRISPR J. 2019. PMID: 31746634 Free PMC article.
-
Gene Editing by Extracellular Vesicles.Int J Mol Sci. 2020 Oct 5;21(19):7362. doi: 10.3390/ijms21197362. Int J Mol Sci. 2020. PMID: 33028045 Free PMC article. Review.
-
Regulating reproductive genetic services: dealing with spiral-shaped processes and techno-scientific imaginaries.J Assist Reprod Genet. 2021 Feb;38(2):305-317. doi: 10.1007/s10815-020-02017-9. Epub 2021 Jan 6. J Assist Reprod Genet. 2021. PMID: 33405005 Free PMC article.
-
Intergenerational monitoring in clinical trials of germline gene editing.J Med Ethics. 2020 Mar;46(3):183-187. doi: 10.1136/medethics-2019-105620. Epub 2019 Aug 31. J Med Ethics. 2020. PMID: 31473657 Free PMC article.
References
-
- Baltimore D., Berg P., Botchan M., Carroll D., Charo R. A., Church G., Corn J. E., Daley G. Q., Doudna J. A., Fenner M., Greely H. T., Jinek M., Martin G. S., Penhoet E., Puck J., Sternberg S. H., Weissman J. S., Yamamoto K. R. A prudent path forward for genomic engineering and germline gene modification. Science. 2015;348(6230):36–38. - PMC - PubMed
-
- Bosley K. S., Botchan M., Bredenoord A. L., Carroll D., Charo R. A., Charpentier E., Cohen R., Corn J., Doudna J., Feng G., Greely H. T., Isasi R., Ji W., Kim J. S., Knoppers B., Lanphier E., Li J., Lovell-Badge R., Martin G. S., Moreno J., Naldini L., Pera M., Perry A. C., Venter J. C., Zhang F., Zhou Q. CRISPR germline engineering—The community speaks. Nature Biotechnology. 2015;33(5):478–86. - PubMed
-
- Collins F. S. Statement on NIH funding of research using gene-editing technologies in human embryos. National Institutes of Health, Department of Health and Human Services; 2015.
-
- Food and Drug Administration FDA guidance for industry: Gene therapy clinical trials—Observing subjects for delayed adverse events 2006
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical