In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf
- PMID: 26632446
- PMCID: PMC5528727
- DOI: 10.1016/j.molonc.2015.10.016
In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf
Abstract
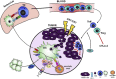
As cancer immunotherapy continues to benefit from novel approaches which cut immune 'brake pedals' (e.g. anti-PD1 and anti-CTLA4 antibodies) and push immune cell gas pedals (e.g. IL2, and IFNα) there will be increasing need to develop immune 'steering wheels' such as vaccines to guide the immune system specifically toward tumor associated antigens. Two primary hurdles in cancer vaccines have been: identification of universal antigens to be used in 'off-the-shelf' vaccines for common cancers, and 2) logistical hurdles of ex vivo production of individualized whole tumor cell vaccines. Here we summarize approaches using 'in situ vaccination' in which intratumoral administration of off-the-shelf immunomodulators have been developed to specifically induce (or amplify) T cell responses to each patient's individual tumor. Clinical studies have confirmed the induction of systemic immune and clinical responses to such approaches and preclinical models have suggested ways to further potentiate the translation of in situ vaccine trials for our patients.
Keywords: Cancer immunotherapy; Checkpoint blockade; Dendritic cells; In situ vaccination; Oncolytic viruses; Toll like receptors.
Copyright © 2015 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Andtbacka, R.H. , Kaufman, H.L. , Collichio, F. , Amatruda, T. , Senzer, N. , Chesney, J. , Delman, K.A. , Spitler, L.E. , Puzanov, I. , Agarwala, S.S. , Milhem, M. , Cranmer, L. , Curti, B. , Lewis, K. , Ross, M. , Guthrie, T. , Linette, G.P. , Daniels, G.A. , Harrington, K. , Middleton, M.R. , Miller, W.H. , Zager, J.S. , Ye, Y. , Yao, B. , Li, A. , Doleman, S. , VanderWalde, A. , Gansert, J. , Coffin, R.S. , 2015. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, (25) 2780–2788. 10.1200/JCO.2014.58.3377 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

