Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients
- PMID: 26633102
- PMCID: PMC10986644
- DOI: 10.1002/14651858.CD007746.pub2
Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients
Abstract
Background: Modern immunosuppressive regimens after kidney transplantation usually use a combination of two or three agents of different classes to prevent rejection and maintain graft function. Most frequently, calcineurin-inhibitors (CNI) are combined with corticosteroids and a proliferation-inhibitor, either azathioprine (AZA) or mycophenolic acid (MPA). MPA has largely replaced AZA as a first line agent in primary immunosuppression, as MPA is believed to be of stronger immunosuppressive potency than AZA. However, treatment with MPA is more costly, which calls for a comprehensive assessment of the comparative effects of the two drugs.
Objectives: This review of randomised controlled trials (RCTs) aimed to look at the benefits and harms of MPA versus AZA in primary immunosuppressive regimens after kidney transplantation. Both agents were compared regarding their efficacy for maintaining graft and patient survival, prevention of acute rejection, maintaining graft function, and their safety, including infections, malignancies and other adverse events. Furthermore, we investigated potential effect modifiers, such as transplantation era and the concomitant immunosuppressive regimen in detail.
Search methods: We searched Cochrane Kidney and Transplant's Specialised Register (to 21 September 2015) through contact with the Trials' Search Co-ordinator using search terms relevant to this review.
Selection criteria: All RCTs about MPA versus AZA in primary immunosuppression after kidney transplantation were included, without restriction on language or publication type.
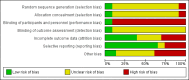
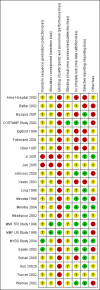
Data collection and analysis: Two authors independently determined study eligibility, assessed risk of bias and extracted data from each study. Statistical analyses were performed using the random-effects model and the results were expressed as risk ratio (RR) for dichotomous outcomes and mean difference (MD) for continuous outcomes with 95% confidence intervals (CI).
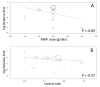
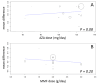
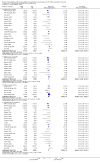
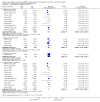
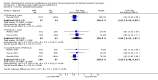
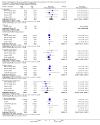
Main results: We included 23 studies (94 reports) that involved 3301 participants. All studies tested mycophenolate mofetil (MMF), an MPA, and 22 studies reported at least one outcome relevant for this review. Assessment of methodological quality indicated that important information on factors used to judge susceptibility for bias was infrequently and inconsistently reported.MMF treatment reduced the risk for graft loss including death (RR 0.82, 95% CI 0.67 to 1.0) and for death-censored graft loss (RR 0.78, 95% CI 0.62 to 0.99, P < 0.05). No statistically significant difference for MMF versus AZA treatment was found for all-cause mortality (16 studies, 2987 participants: RR 0.95, 95% CI 0.70 to 1.29). The risk for any acute rejection (22 studies, 3301 participants: RR 0.65, 95% CI 0.57 to 0.73, P < 0.01), biopsy-proven acute rejection (12 studies, 2696 participants: RR 0.59, 95% CI 0.52 to 0.68) and antibody-treated acute rejection (15 studies, 2914 participants: RR 0.48, 95% CI 0.36 to 0.65, P < 0.01) were reduced in MMF treated patients. Meta-regression analyses suggested that the magnitude of risk reduction of acute rejection may be dependent on the control rate (relative risk reduction (RRR) 0.34, 95% CI 0.10 to 1.09, P = 0.08), AZA dose (RRR 1.01, 95% CI 1.00 to 1.01, P = 0.10) and the use of cyclosporin A micro-emulsion (RRR 1.27, 95% CI 0.98 to 1.65, P = 0.07). Pooled analyses failed to show a significant and meaningful difference between MMF and AZA in kidney function measures.Data on malignancies and infections were sparse, except for cytomegalovirus (CMV) infections. The risk for CMV viraemia/syndrome (13 studies, 2880 participants: RR 1.06, 95% CI 0.85 to 1.32) was not statistically significantly different between MMF and AZA treated patients, whereas the likelihood of tissue-invasive CMV disease was greater with MMF therapy (7 studies, 1510 participants: RR 1.70, 95% CI 1.10 to 2.61). Adverse event profiles varied: gastrointestinal symptoms were more likely in MMF treated patients and thrombocytopenia and elevated liver enzymes were more common in AZA treatment.
Authors' conclusions: MMF was superior to AZA for improvement of graft survival and prevention of acute rejection after kidney transplantation. These benefits must be weighed against potential harms such as tissue-invasive CMV disease. However, assessment of the evidence on safety outcomes was limited due to rare events in the observation periods of the studies (e.g. malignancies) and inconsistent reporting and definitions (e.g. infections, adverse events). Thus, balancing benefits and harms of the two drugs remains a major task of the transplant physician to decide which agent the individual patient should be started on.
Conflict of interest statement
In the past 5 years, MW received travel grants from Genzyme
In the past 5 years, CS received grants from Blue Cross Blue Shield, TEVA Pharma and Glaxo Smith Kline
AE, AW, EB and KU do not have any known conflicts of interest
Figures













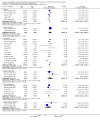






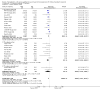



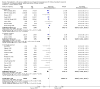



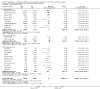





Update of
- doi: 10.1002/14651858.CD007746
References
References to studies included in this review
Army Hospital 2002 {published data only}
-
- Army Hospital (R&R). Mycophenolate versus AZA‐in‐de‐nova renal transplantation ‐ a short term study [abstract]. Indian Journal of Nephrology 2002;12(4):226. [CENTRAL: CN‐00460299]
Baltar 2002 {published data only}
-
- Baltar J, Ortega F, Rebollo P, Gomez E, Laures A, Alvarez‐Grande J. Changes in health‐related quality of life in the first year of kidney transplantation [Cambios en la calidad de vida relacionada con la salud durante el primer ano del trasplante renal]. Nefrologia 2002;22(3):262‐8. [MEDLINE: ] - PubMed
-
- Baltar J, Ortega F, Rebollo P, Rodriguez M, Alvarez‐Grande J. Health related quality of life (HRQOL) in two inmunosuppressor therapy regimens [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A274. [CENTRAL: CN‐00483117]
Busque 2001 {published data only}
-
- Busque S, Shoker A, Landsberg D, McAlister V, Halloran P, Shapiro J. Canadian multicentre kidney trial of prograf/AZA vs. neoral/MMF [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00433620]
-
- Busque S, Shoker A, Landsberg D, McAlister V, Halloran P, Shapiro J. Canadian multicentre trial of prograf/AZA vs. prograf/MMF vs. Neoral/MMF in renal transplantation [abstract]. Transplantation 2000;69(8 Suppl):S114.
-
- Busque S, Shoker A, Landsberg D, McAlister V, Halloran P, Shapiro J, et al. Canadian multicentre trial of tacrolimus/azathioprine/steroids versus tacrolimus/mycophenolate mofetil/steroids versus neoral/mycophenolate mofetil/steroids in renal transplantation. Transplantation Proceedings 2001;33(1‐2):1266‐7. [MEDLINE: ] - PubMed
COSTAMP Study 2002 {published data only}
-
- Mucha K, Foroncewicz B, Paczek L, Pazik J, Lewandowska D, Krawczyk A, et al. 36‐month follow‐up of 75 renal allograft recipients treated with steroids, tacrolimus, and azathioprine or mycophenolate mofetil. Transplantation Proceedings 2003;35(6):2176‐8. [MEDLINE: ] - PubMed
-
- Wlodarczyk Z, Glyda M, Paczek L, Ostrowski M, Marcinkowski W, Klinger M, et al. Long‐term results of steroid withdrawal following renal transplantation in tacrolimus‐based immunosuppression regimens ‐ results of multicenter study [abstract no: 25]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego, CA. 2004. [CENTRAL: CN‐00550597]
-
- Wlodarczyk Z, Walaszewski J, Perner F, Vitko S, Ostrowski M. Tacrolimus/MMF/steroids compared to tacrolimus/AZA/steroids in renal transplantation: differences in efficacy and feasibility of steroid withdrawal [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00416944]
-
- Wlodarczyk Z, Walaszewski J, Perner F, Vitko S, Ostrowski M, Bachleda P, et al. Freedom from rejection and stable kidney function are excellent criteria for steroid withdrawal in tacrolimus‐treated kidney transplant recipients. Annals of Transplantation 2002;7(3):28‐31. [MEDLINE: ] - PubMed
-
- Wlodarczyk Z, Walaszewski J, Perner F, Vitko S, Ostrowski M, Bachleda P, et al. Steroid withdrawal at 3 months after kidney transplantation: a comparison of two tacrolimus‐based regimens. Transplant International 2005;18(2):157‐62. [MEDLINE: ] - PubMed
Egfjord 1999 {published data only}
-
- Egfjord M, Ladefoged J, Olgaard K. Mycophenolate mofetil (MMF) vs azathioprin (AZA) in combination with prednisone, cyclosporin, and ATGAM for prevention of acute renal graft rejection [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):727A. [CENTRAL: CN‐00550650]
-
- Ladefoged J, Egfjord M, Olgaard K. Open randomized study of mofetil vs azathioprin combined with prednisone and cyclosporin for prevention of acute renal graft rejection [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A275. [CN‐00483826]
Folkmane 2001 {published data only}
-
- Folkmane I, Bicans J, Amerika D, Chapenko S, Murovska M, Rosentals R. Low rate of acute rejection and cytomegalovirus infection in kidney transplant recipients with basiliximab. Transplantation Proceedings 2001;33(7‐8):3209‐10. [MEDLINE: ] - PubMed
-
- Folkmane I, Bicans J, Chapenko S, Murovska M, Rosentals R. Results of renal transplantation with different immunosuppressive regimens. Transplantation Proceedings 2002;34(2):558‐9. [MEDLINE: ] - PubMed
-
- Folkmane I, Chapenko S, Amerika D, Bicans J, Murovska M, Rosentals R. beta‐herpes virus activation after kidney transplantation with mycophenolate mofetil‐based maintenance immunosuppression. Transplantation Proceedings 2001;33(3):2384‐5. [MEDLINE: ] - PubMed
-
- Folkmane I, Chapenko S, Murovska M, Rosental R. Low rate of acute rejection and cytomegalovirus infection in renal transplant recipients with basiliximab [abstract no: 1037]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00400939] - PubMed
Isbel 1997 {published data only}
-
- Isbel NM, Smith KG, Leydon JA, Walker RG, Becker GJ. Mycophenolate mofetil suppresses the humoral response to influenza vaccination in renal transplant recipients [abstract no: P1014]. Nephrology 1997;3(Suppl 1):S327. [CENTRAL: CN‐00460991]
Ji 2001 {published data only}
-
- Ji YL, Yang YQ, Yang GB, Yu XQ, Jiang ZP, Shen QR, et al. The clinical investigation of mycophenolate mofetil for the prevention of acute rejection. Academic Journal of Sun Yat‐Sen University of Medical Sciences 2001;22(3):215‐7.
Joh 2005 {published data only}
-
- Kim SJ, Lee KW, Lee SK, Park JH, Woo DH, Oh HY, et al. The influence of mycophenolate (MMF) and azathioprine (AZA) in the same cadaveric renal transplantation [abstract no: FC5‐01]. Nephrology 2003;8(Suppl 1):A18.
Johnson 2000 {published data only}
-
- Ahsan N, Johnson C, Gonwa T, Halloran P, Stegall M, Hardy M, et al. Randomized trial of tacrolimus plus mycophenolate mofetil or azathioprine versus cyclosporine oral solution (modified) plus mycophenolate mofetil after cadaveric kidney transplantation: results at 2 years. Transplantation 2001;72(2):245‐50. [MEDLINE: ] - PubMed
-
- Ahsan N, Milton S, Hershey P, Johnson C, Gonwa T, Halloran P, et al. Diabetes mellitus with neoral (cyclosporine) vs. prograf (tacrolimus) based regimens after kidney transplant [abstract no: 356]. Transplantation 1998;65(12):S91. [CENTRAL: CN‐00653714]
-
- Gonwa T, Johnson C, Ahsan N, Alfrey EJ, Halloran P, Stegall M, et al. Randomized trial of tacrolimus + mycophenolate mofetil or azathioprine versus cyclosporine + mycophenolate mofetil after cadaveric kidney transplantation: results at three years. Transplantation 2003;75(12):2048‐53. [MEDLINE: ] - PubMed
-
- Gonwa T, Johnson C, Ahsan N, Halloran P, Stegall M, Hardy M, et al. Comparative trial of prograf (tacrolimus) in combination with azathioprine or mycophenolate mofetil vs neoral (cyclosporine) with mycophenolate mofetil after kidney transplantation [abstract no: 930]. Transplantation 1999;67(7):S239. [CN‐00764289] - PubMed
-
- Gonwa TA, FK506/MMF Study Trial Group. Two year follow‐up of a randomized trial of FK506 + MMF vs FK506 + AZA vs CyA + MMF [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00433630]
Keven 2003 {published data only}
-
- Keven K, Sahin M, Kutlay S, Sengul S, Erturk S, Erbay B. Immunoglobulin deficiency in kidney allograft recipients: comparative effects of mycophenolate mofetil and azathioprine [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):646A. - PubMed
-
- Keven K, Sahin M, Kutlay S, Sengul S, Erturk S, Ersoz S, et al. Immunoglobulin deficiency in kidney allograft recipients: comparative effects of mycophenolate mofetil and azathioprine. Transplant Infectious Disease 2003;5(4):181‐6. [MEDLINE: ] - PubMed
Ling 1998 {published data only}
-
- Ling JY, Zhu Y, Shun FK. Combined use of MMF with low dosage of cyclosporine A in renal transplantation. Chinese Journal of Organ Transplantation 1998;19(3):175‐6.
Mendez 1998 {published data only}
-
- Mendez R. FK 506 and mycophenolate mofetil in renal transplant recipients: six‐month results of a multicenter, randomized dose ranging trial. FK 506 MMF Dose‐Ranging Kidney Transplant Study Group. Transplantation Proceedings 1998;30(4):1287‐9. [MEDLINE: ] - PubMed
-
- Miller J. Tacrolimus and mycophenolate mofetil in renal transplant recipients: one year results of a multicenter, randomized dose ranging trial. FK506/MMF Dose‐Ranging Kidney Transplant Study Group. Transplantation Proceedings 1999;31(1‐2):276‐7. [MEDLINE: ] - PubMed
-
- Miller J, FK506/MMF Dose‐Ranging Kidney Transplant Study Group. Tacrolimus and mycophenolate mofetil in renal transplant recipients: six and twelve month results of a multicenter, randomized dose ranging study [abstract no: 752]. Transplantation 1998;65(12):S190. [CENTRAL: CN‐00671869]
-
- Miller J, Mendez R, Pirsch JD, Jensik SC. Safety and efficacy of tacrolimus in combination with mycophenolate mofetil (MMF) in cadaveric renal transplant recipients. FK506/MMF Dose‐Ranging Kidney Transplant Study Group. Transplantation 2000;69(5):875‐80. [MEDLINE: ] - PubMed
Merville 2004 {published data only}
-
- Berge F, Durang D, Merville P, Morel D, Mourad G, Potaux L. Beneficial effect of mycophenolate mofetil on the incidence of chronic allograft nephropathy: a randomized protocol biopsy‐based study [abstract no: 1614]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00602028]
-
- Merville P, Berge F, Deminiere C, Morel D, Chong G, Durand D, et al. Interest of mycophenolate mofetil in the prevention of chronic allograft nephropathy: a randomized biopsy‐based study [abstract no: 1076]. American Journal of Transplantation 2002;2(Suppl 3):409. [CENTRAL: CN‐00416277]
-
- Merville P, Berge F, Deminiere C, Morel D, Chong G, Durand D, et al. Lower incidence of chronic allograft nephropathy at 1 year post‐transplantation in patients treated with mycophenolate mofetil. American Journal of Transplantation 2004;4(11):1769‐75. [MEDLINE: ] - PubMed
Miladipour 2002 {published data only}
-
- Miladipour AH, Ghods AJ, Nejadgashti H. Effect of mycophenolate mofetil on the prevention of acute renal allograft rejection. Transplantation Proceedings 2002;34(6):2089‐90. [MEDLINE: ] - PubMed
MMF TRI Study 1996 {published data only}
-
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation 1996;61(7):1029‐37. [MEDLINE: ] - PubMed
-
- Clayton P, McDonald S, Chapman J, Chadban S. Mycophenolate vs azathioprine for kidney transplantation ‐ 15 year follow‐up of a randomised trial [abstract no: 111]. Immunology & Cell Biology 2011;89(7):A1. [EMBASE: 70655945]
-
- Clayton P, McDonald S, Chapman J, Chadban S. Mycophenolate vs azathioprine for kidney transplantation: 15 year follow‐up of a randomized trial [abstract no: 172]. Nephrology 2011;16(Suppl 1):69. [EMBASE: 70532520]
-
- Clayton PA, McDonald SP, Chapman JR, Chadban SJ. Mycophenolate versus azathioprine for kidney transplantation: a 15‐year follow‐up of a randomized trial. Transplantation 2012;94(2):152‐8. [MEDLINE: ] - PubMed
-
- Halloran P, Mathew T, Tomlanovich S, Groth C, Hooftman L, Barker C. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double‐blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. [erratum appears in Transplantation 1997 Feb 27;63(4):618]. Transplantation 1997;63(1):39‐47. [MEDLINE: ] - PubMed
MMF US Study 1995 {published data only}
-
- Mycophenolate mofetil for the prevention of acute rejection of primary cadaveric kidney transplants: status of the MYC 1866 study at 1 year. The U.S. Mycophenolate Mofetil Study Group. Transplantation Proceedings 1997;29(1‐2):348‐9. [MEDLINE: ] - PubMed
-
- Mycophenolate mofetil in cadaveric renal transplantation. US Renal Transplant Mycophenolate Mofetil Study Group. American Journal of Kidney Diseases 1999;34(2):296‐303. [MEDLINE: ] - PubMed
-
- Ferguson RM, US Renal Transplant Mycophenolate Mofetil Study Group. Efficacy of mycophenolate mofetil for the prevention of acute rejection in first cadaveric renal transplantation. [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:335.
-
- Gonwa TA, US Renal Transplant Mycophenolate Mofetil Study Group. Safety of mycophenolate mofetil (MMF) vs azathioprine (AZA) in primary cadaveric renal transplant (Cad TX) [abstract]. 14th Annual Meeting American Society of Transplant Physicians (ASTP); 1995 May 10‐14;Chicago (IL). 1995.
-
- Halloran P, Mathew T, Tomlanovich S, Groth C, Hooftman L, Barker C. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double‐blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. [erratum appears in Transplantation 1997 Feb 27;63(4):618]. Transplantation 1997;63(1):39‐47. [MEDLINE: ] - PubMed
MYSS Study 2004 {published data only}
-
- Gotti E, Perico N, Gaspari F, Cattaneo D, Lesti MD, Ruggenenti P, et al. Blood cyclosporine level soon after kidney transplantation is a major determinant of rejection: insights from the Mycophenolate Steroid‐Sparing Trial. Transplantation Proceedings 2005;37(5):2037‐40. [MEDLINE: ] - PubMed
-
- Perico N, Ruggenenti P, Gotti E, Gaspari F, Cattaneo D, Valente U, et al. In renal transplantation blood cyclosporine levels soon after surgery act as a major determinant of rejection: insights from the MY.S.S. trial. Kidney International 2004;65(3):1084‐90. [MEDLINE: ] - PubMed
-
- Perico N, Ruggenenti P, Gotti E, Gaspari F, Cattaneo D, Valente U, et al. In renal transplantation low blood cyclosporine levels soon after surgery is a determinant of rejection: insights from the MY.S.S. trial [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):11A. [CENTRAL: CN‐00601980]
-
- Remuzzi G, Cravedi P, Costantini M, Lesti M, Ganeva M, Gherardi G, et al. Mycophenolate mofetil versus azathioprine for prevention of chronic allograft dysfunction in renal transplantation: the MYSS follow‐up randomized, controlled clinical trial. Journal of the American Society of Nephrology 2007;18(6):1973‐85. [MEDLINE: ] - PubMed
-
- Remuzzi G, Lesti M, Gotti E, Ganeva M, Dimitrov BD, Ene‐Iordache B, et al. Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomised trial. Lancet 2004;364(9433):503‐12. [MEDLINE: ] - PubMed
Sadek 2002 {published data only}
-
- Sadek S, Medina J, Arias M, Sennesael J, Squifflet JP, Vogt B, et al. Short‐term combination of mycophenolate mofetil with cyclosporine as a therapeutic option for renal transplant recipients: a prospective, multicenter, randomized study. Transplantation 2002;74(4):511‐7. [MEDLINE: ] - PubMed
-
- Sadek S, Vogt B, Beauregard‐Zllinger L, Prestele H, Neoral Phase IV Study Group. Short‐term combination of mycophenolate mofetil with cyclosporine as a safe therapeutic option for renal transplant recipients [abstract]. Transplantation 2000;69(8 Suppl):S160. [CENTRAL: CN‐00447538] - PubMed
-
- Sadek SA, Vogt B, Beauregard‐Zollinger L, Neoral Phase IV Study Group. Short‐term mycophenolate mofetil combined with cyclosporine compared to standard maintenance regimens of cyclosporine with mycophenolate mofetil or azathioprine in kidney transplantation: interim analysis [abstract no: 927]. Transplantation 1999;67(7):S238. [CENTRAL: CN‐00766427]
-
- Vogt B, Sadek S, Phase IV Neonatal Study Group. Short‐term combination of mycophenolate mofetil with cyclosporine as a safe therapeutive option in renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00448227]
Suhail 2000 {published data only}
-
- Suhail SM, Vathsala A, Lou HX, Woo KT. Safety and efficacy of mycophenolate mofetil for prophylaxis in Asian renal transplant recipients. Transplantation Proceedings 2000;32(7):1757‐8. [MEDLINE: ] - PubMed
-
- Vathsala A, Lou H, Suhail SM, Woo K. Does 6 months prophylaxis with mycophenolate mofetil reduce acute rejection in renal transplantation? [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):711A. [CENTRAL: CN‐00583822]
Sun 2002b {published data only}
-
- Sun CC, Hao JW, Sun J, Yang DA. A comparison between therapeutic effects of mycophenolate mofetil and azathioprine in the management of patients after renal transplantation. Yiyao Dao Bao [Herald of Medicine] 2002;21(9):544‐6. [CENTRAL: CN‐00429261]
Tuncer 2002 {published data only}
-
- Tuncer M, Gurkan A, Erdogan O, Demirbas A, Suleymanlar G, Ersoy FF, et al. Mycophenolate mofetil in renal transplantation: five years experience. Transplantation Proceedings 2002;34(6):2087‐8. [MEDLINE: ] - PubMed
Weimer 2002 {published data only}
-
- Weimer R, Deisz S, Dietrich H, Renner F, Bodeker RH, Daniel V, et al. Impact of maintenance immunosuppressive regimens‐‐balance between graft protective suppression of immune functions and a near physiological immune response. Transplant International 2011;24(6):596‐609. [MEDLINE: ] - PubMed
-
- Weimer R, Deisz S, Dietrich H, Yildiz S, Staak A, Renner F, et al. Impact of maintenance immunosuppressive regimens on immunological parameters of graft outcome [abstract no: P101]. Transplant International 2007;20(Suppl 2):120.
-
- Weimer R, Deisz S, Renner F, Dietrich H, Daniel V, Kamali‐Ernst S, et al. Different impact of maintenance immunosuppressive regimens on the immune response of renal transplant recipients [abstract no: 112]. Transplantation 2008;86(2 Suppl):40. [CENTRAL: CN‐00671788]
-
- Weimer R, Deisz S, Renner F, Dietrich H, Suesal C, Kamali‐Ernst S, et al. Impact of steroid withdrawal on the immune response of renal transplant recipients [abstract no: O‐249]. Transplant International 2009;22(Suppl 2):66.
-
- Weimer R, Ettrich M, Renner F, Dietrich H, Susal C, Deisz S, et al. ATG induction in renal transplant recipients: Long‐term hazard of severe infection is associated with long‐term functional T cell impairment but not the ATG‐induced CD4 cell decline. Human Immunology 2014;75(6):561‐9. [MEDLINE: ] - PubMed
References to studies excluded from this review
Araujo 1999 {published data only}
-
- Araujo MR, Oliveira AC, Abensur H, Marcondes M, Romao JE, Zatz R, et al. Mycopheolate mofetil (MMF) in the treatment of chronic renal allograft rejection: a three‐year follow‐up [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):719A. [CENTRAL: CN‐00550469]
Asci 2002 {published data only}
-
- Asci G, Toz H, Ok E, Sezis M, Basci A. No benefit from mycophenolate mofetil in renal transplant recipients with chronic allograft nephropathy [abstract no: O56]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):18. [MEDLINE: ]
Baek 2004 {published data only}
-
- Baek H, Huh W, Kim JA, Kim Y, Kim DJ, Oh H, et al. Efficacy of mycophenolate mofetil and azathiporine therapy in paired cadaveric renal transplantation: five‐year experience [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:402. [CENTRAL: CN‐00509077]
Bataille 2010 {published data only}
-
- Bataille S, Moal V, Gaudart J, Indreies M, Purgus R, Dussol B, et al. Cytomegalovirus risk factors in renal transplantation with modern immunosuppression. Transplant Infectious Disease 2010;12(6):480‐8. [MEDLINE: ] - PubMed
Benfield 1999 {published data only}
-
- Benfield MR, Herrin J, Feld L, Rose S, Stablein D, Tejani A. Safety of kidney biopsy in pediatric transplantation: a report of the Controlled Clinical Trials in Pediatric Transplantation Trial of Induction Therapy Study Group. Transplantation 1999;67(4):544‐7. [MEDLINE: ] - PubMed
-
- Benfield MR, Symons JM, Bynon S, Eckhoff D, Herrin J, Harmon W, et al. Mycophenolate mofetil in pediatric renal transplantation. Pediatric Transplantation 1999;3(1):33‐7. [MEDLINE: ] - PubMed
-
- Benfield MR, Tejani A, Harmon WE, McDonald R, Stablein DM, McIntosh M, et al. A randomized multicenter trial of OKT3 mAbs induction compared with intravenous cyclosporine in pediatric renal transplantation. Pediatric Transplantation 2005;9(3):282‐92. [MEDLINE: ] - PubMed
-
- Tejani A. A randomized prospective multicenter trial of T‐cell antibody induction therapy in pediatric renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000.
-
- Tejani A, Harmon W, Benfield M, Elshihabi I, McDonald R, Stablein D, et al. A randomized prospective multicenter trial of T‐cell antibody induction therapy in pediatric renal transplantation [abstract]. Transplantation 2000;69(8 Suppl):S111. [CENTRAL: CN‐00402826]
Boletis 1999b {published data only}
-
- Boletis JN, Stamatiadis D, Markis F, Konstandinidou I, Theodosis I, Mansour M, et al. Mycophenolate mofetil in renal transplantation [abstract]. Nephrology Dialysis Transplantation 1999;14:2975. [CENTRAL: CN‐00278914]
Brennan 2005 {published data only}
-
- Agha IA, Hardinger KL, Bohl D, Ansari A, Dyk P, Koch M, et al. Preemptive withdrawal of AZA or MMF prevents progression of BK viremia to BK nephropathy: a prospective randomized controlled trial of BK virus infection after renal transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):200. [CENTRAL: CN‐00509045]
-
- Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault‐Keener M, Schnitzler MA, Major EO, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. American Journal of Transplantation 2005;5(9):2213‐21. [MEDLINE: ] - PubMed
-
- Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction.[erratum appears in Am J Transplant. 2005 Apr;5(4 Pt 1):839]. American Journal of Transplantation 2005;5(3):582‐94. [MEDLINE: ] - PubMed
-
- Brennan DC, Bohl DL, Storch GA, Major EO, Agha IA, Schnitzler MA. High donor antibody level and HLA C7 predict sustained BK‐polyoma viremia: results of a randomized prospective trial [abstract no: P817]. Transplantation 2004;78(2 Suppl):488. [CENTRAL: CN‐00644222]
-
- Hardinger KL, Bohl DL, Schnitzler MA, Lockwood M, Storch GA, Brennan DC. A randomized, prospective, pharmacoeconomic trial of tacrolimus versus cyclosporine in combination with thymoglobulin in renal transplant recipients. Transplantation 2005;80(1):41‐6. [MEDLINE: ] - PubMed
Cransberg 2007 {published data only}
-
- Cransberg K, Cornelissen M, Lilien M, Hoeck K, Davin JC, Nauta J. Maintenance immunosuppression with mycophenolate mofetil and corticosteroids in pediatric kidney transplantation: temporary benefit but not without risk. Transplantation 2007;83(8):1041‐7. [MEDLINE: ] - PubMed
El‐Agroudy 2009 {published data only}
-
- El‐Agroudy AE, El‐Dahshan KF, Wafa EW, Sheashaa HA, Gad ZA, Ismail AM, et al. Safe conversion of mycophenolate mofetil to azathioprine in kidney transplant recipients with sirolimus‐based immunosuppression. Nephrology 2009;14(2):255‐61. [MEDLINE: ] - PubMed
-
- El‐Dahshan K, El‐Agroudy A, Wafa E, Gad Z. Safe conversion of mycophenolate mofetil to azathioprine in kidney transplant recipients with sirolimus‐based immunosuppression [abstract no: Su711]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. - PubMed
Ettenger 1995 {published data only}
-
- Ettenger R, Mentser BW, Potter D, Cohen A. Mycophenolate mofetil (MMF) in pediatric (PED) renal transplantation (TX): a report of the PED MMF study group [abstract]. Journal of the American Society of Nephrology 1995;6(3):1082. [CENTRAL: CN‐00483891]
Griffin 2003 {published data only}
-
- Griffin MD, Slezak JM, Kozel TK, Bergstralh EJ, Schwab TR, Gloor JM, et al. Renal function loss in chronic allograft nephropathy: results of a two‐year study of mycophenolate mofetil substitution for azathioprine [abstract]. American Journal of Transplantation 2003;3(Suppl 5):223. [CENTRAL: CN‐00445554]
Ha 2004 {published data only}
-
- Ha J, Yun IJ, Lee JH, Choi SJ, Kang JM, Kim SJ. Azathioprine combination therapy can stably reduce cyclosporine dose as mycophenolate mofetil combination therapy for the recipients showing stable renal function after renal transplantation [abstract no: P‐19]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00583458]
Hernandez 2007 {published data only}
-
- Hernandez D, Miquel R, Porrini E, Fernandez A, Gonzalez‐Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine‐based immunosuppression. Transplantation 2007;84(6):706‐14. [MEDLINE: ] - PubMed
Jain 2001 {published data only}
-
- Jain S, Metcalfe M, White SA, Furness PN, Nicholson ML. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without mycophenolate mofetil. Transplantation Proceedings 2001;33(3):2165‐6. [MEDLINE: ] - PubMed
-
- Jain S, Metcalfe M, White SA, Furness PN, Nicholson ML. Randomized trial comparing mycophenolate mofetil and azathioprine to allow cyclosporin reduction in chronic allograft nephropathy [abstract no: PO513W]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00445890]
-
- Nicholson ML, Jain S, Metcalfe M, White SA, Furness PN. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without mycophenolate mofetil [abstract no: 437]. Transplantation 2000;69(8 Suppl):S227. [CENTRAL: CN‐00446949] - PubMed
Jirasiritham 2000 {published data only}
-
- Jirasiritham S, Sumethkul V, Mavichak V, Chalermsanyakorn P. The treatment of chronic rejection with mycophenolate mofetil versus azathioprine in kidney transplantation. Transplantation Proceedings 2000;32(7):2040‐2. [MEDLINE: ] - PubMed
Kasiske 1997 {published data only}
-
- Kasiske BL, Johnson HJ, Heim‐Duthoy KL, Rao VK, Dahl DC, Jacobs DM, et al. Does mycophenolate mofetil improve already good results from cyclosporine induction early after renal transplantation? [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:236. [CENTRAL: CN‐00509263]
Khosroshahi 2006a {published data only}
-
- Khosroshahi HT, Asghari A, Estakhr R, Baiaz B, Ardalan MR, Shoja MM. Effects of azathioprine and mycophenolate mofetil‐immunosuppressive regimens on the erythropoietic system of renal transplant recipients. Transplantation Proceedings 2006;38(7):2077‐9. [MEDLINE: ] - PubMed
-
- Khosroshahi HT, Estakhri R, Shoja MM. Effects of azathioprine and mycophenolate mofetil based immunosuppressive regimen on the erthropoietic system of the renal transplant recipients [abstract no: SP735]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv263. [CENTRAL: CN‐00626063] - PubMed
Kim 1999 {published data only}
-
- Kim JK, Shin YH, Park YG, Hur D, Kim MS, Lee SR. Clinical observation of mycophenolate mofetil for the prevention of acute rejection in renal transplantation [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):734A. [CENTRAL: CN‐00550544]
Langman 1996 {published data only}
-
- Langman LJ, LeGatt DF, Halloran PF, Yatscoff RW. Pharmacodynamic assessment of mycophenolic acid‐induced immunosuppression in renal transplant recipients. Transplantation 1996;62(5):666‐72. [MEDLINE: ] - PubMed
Lezaic 2005 {published data only}
-
- Lezaic V, Marinkovic J, Ristic S, Dokic Z, Radivojevic D, Blagojevic R, et al. Conversion of azathioprine to mycophenolate mofetil and chronic graft failure progression [abstract]. Transplantation 2004;78(2 Suppl):268. [CENTRAL: CN‐00509315] - PubMed
-
- Lezaic VD, Marinkovic J, Ristic S, Dokic ZM, Basta Jovanovic G, Radivojevic DM, et al. Conversion of azathioprine to mycophenolate mofetil and chronic graft failure progression. Transplantation Proceedings 2005;37(2):734‐6. [MEDLINE: ] - PubMed
Lison 2004 {published data only}
-
- Eilts V, Zantvoort F, Wullstein HG, Hillebrand GF, Gobmann J, Kachel HG, et al. Mycophenolate mofetil ‐ a solution for cyclosporine induced hypertension in kidney transplanted patients? [abstract no: T‐PO50008]. Nephrology 2005;10(Suppl 1):A209. [CENTRAL: CN‐00583349]
-
- Lison AE, Eilts V, Zantvoort F, Wullstein H. Mycophenolate mofetil‐ a solution for cyclosporine induced hypertension in kidney transplanted patients? [abstract]. Transplantation 2004;78(2 Suppl):659. [CENTRAL: CN‐00527138]
Makhdoomi 2005 {published data only}
-
- Makhdoomi K, Ahmadpoor P, Ghafari A, Yekta Z. Comparison of 1 year allograft function with mycofenolate mofetil versus azathioprine in renal transplant patients [abstract no: T‐PO50033]. Nephrology 2005;10(Suppl):A216.
Mandelbaum 1998 {published data only}
-
- Mandelbaum AP, Wiesel M, Ksoll‐Ruddek D, Zeier MG. Long‐term results of mycophenolate‐mofetil (MMF) as compared to azathioprine (AZA) in renal transplantation [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):686A. [CENTRAL: CN‐00446574]
Merion 2000 {published data only}
-
- Merion RM, Henry ML, Melzer JS, Sollinger HW, Sutherland DE, Taylor RJ. Randomized, prospective trial of mycophenolate mofetil versus azathioprine for prevention of acute renal allograft rejection after simultaneous kidney‐pancreas transplantation. Transplantation 2000;70(1):105‐11. [MEDLINE: ] - PubMed
Metcalfe 2002 {published data only}
-
- Brook NR, Metcalf MS, Jain S, Bicknell GR, Nicholson ML, Harper SJ. A randomised trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy [abstract no: P‐97]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550372] - PubMed
-
- Brook NR, Metcalfe MS, Waller JR, Jain S, Hosgood SA, Nicholson ML. A prospective randomised trial of mycophenolate mofetil and azathioprine after calcineurin reduction in renal allografts with established chronic allograft nephropathy [abstract]. American Journal of Transplantation 2004;4(Suppl 8):485. [CENTRAL: CN‐00509106]
-
- Metcalfe MS, Jain S, Waller JR, Saunders RN, Bicknell GR, Nicholson ML. A randomized trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy. Transplantation Proceedings 2002;34(5):1812‐4. [MEDLINE: ] - PubMed
MMF 1998 {published data only}
-
- Mycophenolate mofetil for the treatment of a first acute renal allograft rejection: The Mycophenolate Mofetil Acute Renal Rejection Study Group.[erratum appears in Transplantation 1998 Apr 15;65(7):followi]. Transplantation 1998;65(2):235‐41. [MEDLINE: ] - PubMed
-
- Mycophenolate Mofetil Acute Renal Rejection Study Group. Mycophenolate mofetil for the treatment of a first acute renal allograft rejection: three‐year follow‐up. The Mycophenolate Mofetil Acute Renal Rejection Study Group. Transplantation 2001 Apr 27;71(8):1091‐7. [MEDLINE: ] - PubMed
-
- Pescovitz MD. Mycophenolate mofetil for the treatment of renal transplant rejection: 3 years of follow‐up [abstract no: 929]. Transplantation 1999;67(7):S239. [CENTRAL: CN‐00765711]
-
- Pirsch J, Best JH, 1912 Renal Transplant Mycophenolate Mofetil Study Group. An economic evaluation of mycophenolate mofetil (MMF) versus azathioprine (AZA) as adjunctive treatment for acute renal allograft rejection [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (IL). 1997:239. [CENTRAL: CN‐00509418]
-
- Pirsch JD, Pescovitz MD, Ferguson R, Deierhoi M, Hooftman L, Navarro M. Mycophenolate mofetil for the treatment of first acute renal allograft rejection [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (IL). 1997:261. [CENTRAL: CN‐00509419]
MO2ART Study 2003 {published data only}
-
- Balshaw R, Marra C, Nashan B, Hagenmeyer EG, Kalo Z, Keown P. Two‐hour post‐dose cyclosporine levels in renal transplantation: a cost‐effective strategy for reducing graft rejection [abstract no: 3316]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415222]
-
- Buchler M, Chadban S, Cole E, Midtvedt K, Thervet E, Prestele H, et al. Evolution of the absorption profile of cyclosporine A in renal transplant recipients: a longitudinal study of the de novo and maintenance phases. Nephrology Dialysis Transplantation 2006;21(1):197‐202. [MEDLINE: ] - PubMed
-
- Buchler M, Chadban S, Jost L, Rodriguez A, Beauregard L, Balshaw R. Excellent renal tolerability of neoral C‐2h monitoring in de novo kidney transplantation: initial results of the MO2ART trial [abstract no: 1038]. American Journal of Transplantation 2002;2(Suppl 3):399. [CENTRAL: CN‐00550435]
-
- Chadban S, Pilmore H, Goodman D, Hutchison B, MO2ART SG. Minimal rejection and excellent graft function by neoral C2 monitoring in renal transplantation: interim results of MO2ART [abstract]. Transplantation Society of Australia & New Zealand (TSANZ). 21st Annual Scientific Meeting; 2003 Apr 9‐11; Canberra, Australia. 2003:65. [CENTRAL: CN‐00444732]
-
- Chadban S, Pilmore H, Goodman D, Jose M, Hutchison B, Cole E. Optimal C2 targets after month 3 for renal transplant recipients receiving cyclosporin‐microemulsion based triple therapy ‐ the MO2ART trial [abstract no: 93]. Transplantation Society of Australia & New Zealand (TSANZ). 22nd Annual Scientific Meeting; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:83. [CENTRAL: CN‐00583658]
Nowacka‐Cieciur 2000 {published data only}
-
- Nowacka‐Cieciura E, Kaminska B, Cieciura T, Gradowska L, Pazik J, Lao M, et al. Randomised open clinical trial of conversion from mycophenolate mofetil to azathioprine in cadaveric renal transplantation. Transplant International 2000;13(Suppl 1):S68‐72. [MEDLINE: ] - PubMed
Oliveira 1999 {published data only}
-
- Oliveira JG, Ramos JP, Xavier P, Sampaio S, Magalhaees M, Mandes A, et al. Lymphocyte subsets in peripheral blood and inside the allograft in renal tx treated with AZA versus MMF: CD3DR andICAM‐1 down regulation with MMF [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A276. [CENTRAL: CN‐00583426]
Schurter 1997 {published data only}
-
- Schurter G, Glicklich D, Greenstein SM, Schreiber T, Mallis M, Clemetson S, et al. Mycophenolate mofetil (MMF) therapy for chronic rejection in renal transplant recipients [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (IL). 1997:133. [CENTRAL: CN‐00509469]
Smak Gregoor 2000 {published data only}
-
- Smak Gregoor PJ, Gelder T, Besouw NM, mast P, kuiper P, Ijzermans JN, et al. Long‐term follow‐up of a prospective, randomised study on minimising immunosuppressive medication from one year after kidney transplantation [abstract no: 0016]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416672]
-
- Smak Gregoor PJ, Gelder T, Besouw NM, Mast BJ, IJzermans JN, Weimar W. Randomized study on the conversion of treatment with cyclosporine to azathioprine or mycophenolate mofetil followed by dose reduction. Transplantation 2000;70(1):143‐8. [MEDLINE: ] - PubMed
-
- Smak Gregoor PJ, Gelder T, Besouw NM, Mast BJ, Kuiper P, IJzermans JN, et al. Five‐year follow‐up of a prospective, randomised study on minimising immunosuppressive medication from one year after kidney transplantation [abstract no: 1276]. American Journal of Transplantation 2003;3(Suppl 5):479. [CENTRAL: CN‐00447778]
-
- Gelder T, Kuiper P, Besouw NM, Mast B, Smak Gregoor PJ, Ijzermans JN, et al. Randomised trial comparing conversion of maintenance treatment with ciclosporine and prednisone to azathioprine or mycophenolate mofetil with prednisone one year after kidney transplantation [abstract no: 248]. Transplantation 1998;65(12):S64. [CENTRAL: CN‐00583809]
Touchard 2005 {published data only}
-
- Touchard G, Bridoux F, Etienne I, Toupance O, Lavaud S, Hurault de Ligny B, et al. Efficacy and safety of maintenance neoral monotherapy compared to bitherapy neoral+MMF or neoral+AZA in renal transplantation [abstract no: 1198]. American Journal of Transplantation 2005;5(Suppl 11):462. [CENTRAL: CN‐00793401]
Tsinalis 2000 {published data only}
-
- Tsinalis D, Binet I, Dickenmann M, Steiger J, Brunner F, Thiel G. Cost of medical care after renal transplantation comparing cyclosporine‐mycophenolate to tacrolimus‐azathioprine ‐ a randomised controlled study [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000.
Vacher‐Coponat 2006 {published data only}
-
- Al‐Massarani G, Vacher‐Coponat H, Paul P, Widemann A, Arnaud L, Loundou A, et al. Impact of immunosuppressive treatment on endothelial biomarkers after kidney transplantation. American Journal of Transplantation 2008;8(11):2360‐7. [MEDLINE: ] - PubMed
-
- Legris T, Picard C, Moal V, Burtey S, Loundou A, Purgus R, et al. Humoral immunity after kidney transplantation: impact of two randomized immunosuppressive protocols. Annals of Transplantation 2013;18:622‐34. [MEDLINE: ] - PubMed
-
- Vacher‐Coponat H, Brunet C, Moal V, Loundou A, Bonnet E, Lyonnet L, et al. Tacrolimus/mycophenolate mofetil improved natural killer lymphocyte reconstitution one year after kidney transplant by reference to cyclosporine/azathioprine. Transplantation 2006;82(4):558‐66. [MEDLINE: ] - PubMed
-
- Vacher‐Coponat H, Indreies M, Moal V, Purgus R, Moussi JF, Dussol B, et al. Cost effectiveness comparison for two immunosuppressive regimens in kidney transplantation [abstract no: 1634]. Transplantation 2008;86(2 Suppl):541. [CENTRAL: CN‐00679019]
-
- Vacher‐Coponat H, Moal V, Indreies M, Purgus R, Loundou A, Burtey S, et al. A randomized trial with steroids and antithymocyte globulins comparing cyclosporine/azathioprine versus tacrolimus/mycophenolate mofetil (CATM2) in renal transplantation. Transplantation 2012;93(4):437‐43. [MEDLINE: ] - PubMed
van der Mast 2000 {published data only}
-
- Mast BJ, Besouw NM, Kuiper P, Vaessen LM, Ijzermans JN, Gelder T, et al. A longitudinal study of TGF‐beta1 protein levels in renal allograft recipients converted from CsA to MMF or AZA. Clinical Transplantation 2000;14(1):66‐9. [MEDLINE: ] - PubMed
Vanrenterghem 1998 {published data only}
-
- Forsythe J. Tacrolimus and mycophenolate mofetil in cadaveric renal transplant recipients. The European Multicentre Tacrolimus/MMF Study Group. Transplantation Proceedings 1999;31(7A):69S‐71S. [MEDLINE: ] - PubMed
-
- Morales JM, Andres A, Morales E, Herrero JC, Cubas A, Praga M, et al. Tacrolimus, mycophenolate mofetil and corticosteroids as primary immunosuppression after renal transplantation at the Hospital 12 de Octubre, Madrid. Transplantation Proceedings 1999;31(7A):75S‐77S. [MEDLINE: ] - PubMed
-
- Squifflet JP, Backman L, Claesson K, Dietl KH, Ekberg H, Forsythe JL, et al. Dose optimization of mycophenolate mofetil when administered with a low dose of tacrolimus in cadaveric renal transplant recipients. Transplantation 2001;72(1):63‐9. [MEDLINE: ] - PubMed
-
- Squifflet JP, Hooff JP, Vanrenterghem Y. The Benelux experience with the combination of tacrolimus and mycophenolate mofetil. Transplantation Proceedings 1999;31(7A):72S‐74S. [MEDLINE: ] - PubMed
-
- Vanrenterghem Y, Squifflet JP, Forsythe J, Heeman U, Backman L, Taube D, et al. Co‐administration of tacrolimus and mycophenolate mofetil in cadaveric renal transplant recipients. Transplantation Proceedings 1998;30(4):1290‐1. [MEDLINE: ] - PubMed
Woeste 2002 {published data only}
-
- Woeste G, Wullstein C, Dette K, Pridohl O, Lubke P, Bechstein WO. Tacrolimus/mycophenolate mofetil vs cyclosporine A/azathioprine after simultaneous pancreas and kidney transplantation: five‐year results of a randomized study. Transplantation Proceedings 2002;34(5):1920‐1. [MEDLINE: ] - PubMed
-
- Woeste G, Wullstein C, Pridohl O, Dette K, Bechstein WO. Five year results of a randomized study comparing FK506/MMF and ciclosporin A/azathioprine after simultaneous pancreas and kidney transplantation [abstract]. 5th International Conference on New Trends in Clinical and Experimental Immunosuppression; 2002 Feb 7‐10; Geneva, Switzerland. 2002. [CENTRAL: CN‐00817724]
Wuthrich 2000 {published data only}
-
- Binswanger U, Ambuehl P, Cicvara Muzar S, Knoflach A. Randomized conversion from Mycophenolate to Azathioprine: follow‐up after 2 years. [abstract no: 1592]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00602119]
-
- Inderbitzin M, Muzar SC, Ambuhl PM, Knoflach A, Binswanger U. Randomized conversion from mycophenolate mofetil to azathioprine: follow‐up after 2 years [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):896A. [CENTRAL: CN‐00602120]
-
- Wuthrich RP, Cicvara S, Ambuhl PM, Binswanger U. Randomized trial of conversion from mycophenolate mofetil to azathioprine 6 months after renal allograft transplantation. Nephrology Dialysis Transplantation 2000;15(8):1228‐31. [MEDLINE: ] - PubMed
References to studies awaiting assessment
Do 2001a {published data only}
-
- Do JH, Huh W, Kim JA, Lee HR, Choi SC, Han HJ. The influence of mycophenolate (MMF) and azathioprine (AZA) in the same cadaveric renal transplantation. Korean Journal of Nephrology 2001;20(6):949‐54. [CENTRAL: CN‐01044485]
References to ongoing studies
ATHENA Study 2012 {published data only}
-
- Perico N. A randomized, prospective, multicenter trial to compare the effect on chronic allograft nephropathy prevention of mycophenolate mofetil versus azathioprine as the sole immunosuppressive therapy for kidney transplant recipients. www.clinicaltrials.gov/ct2/show/NCT00494741 (accessed 30 November 2015).
Additional references
Atkins 2004
Budde 2004
-
- Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, et al. Enteric‐coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1‐year study. American Journal of Transplantation 2004;4(2):237‐43. [MEDLINE: ] - PubMed
Domhan 2009
-
- Domhan S, Zeier M, Abdollahi A. Immunosuppressive therapy and post‐transplant malignancy. Nephrology Dialysis Transplantation 2009;24(4):1097‐103. [MEDLINE: ] - PubMed
Ekberg 2003
-
- Ekberg H. Graft survival benefit to be expected of new immunosuppressive regimens. Transplantation Reviews 2003;17(4):187‐93. [EMBASE: 2003503607]
European MMF Study Group 1995
-
- Placebo‐controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. European Mycophenolate Mofetil Cooperative Study Group. Lancet 1995;345(8961):1321‐5. [MEDLINE: ] - PubMed
FDA 2008
-
- U.S Food, Drug Administration. CellCept (mycophenolate mofetil capsules) FDA Medwatch. 2008. http://www.fda.gov/safety/medwatch/safetyinformation/safety‐relateddrugl... (accessed 4 November 2015).
Halloran 2004
-
- Halloran PF. Immunosuppressive drugs for kidney transplantation. New England Journal of Medicine 2004;351(26):2715‐29. [MEDLINE: ] - PubMed
Hardinger 2013
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ioannidis 2004
-
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [MEDLINE: ] - PubMed
Israni 2010
-
- Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. American Journal of Transplantation 2010;10(2):338‐53. [MEDLINE: ] - PubMed
Johnston 2010
-
- Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation 2010;89(9):1057‐70. [MEDLINE: ] - PubMed
Kauffman 2006
-
- Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Post‐transplant de novo malignancies in renal transplant recipients: the past and present. Transplant International 2006;19(8):607‐20. [MEDLINE: ] - PubMed
KDIGO 2009
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation 2009;9 Suppl 3:S1‐155. [MEDLINE: ] - PubMed
Kent 2007
-
- Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA 2007;298(10):1209‐12. [MEDLINE: ] - PubMed
Knight 2009
-
- Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 2009;87(6):785‐94. [MEDLINE: ] - PubMed
Lau 2006
Marcen 2009
-
- Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 2009;69(16):2227‐43. [MEDLINE: ] - PubMed
Meier‐Kriesche 2003
-
- Meier‐Kriesche HU, Steffen BJ, Hochberg AM, Gordon RD, Liebman MN, Morris JA, et al. Mycophenolate mofetil versus azathioprine therapy is associated with a significant protection against long‐term renal allograft function deterioration. Transplantation 2003;75(8):1341‐6. [MEDLINE: ] - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [MEDLINE: ] - PubMed
Morath 2004
-
- Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M. Malignancy in renal transplantation. Journal of the American Society of Nephrology 2004;15(6):1582‐8. [MEDLINE: ] - PubMed
Mowbray 1965
Pascual 2002
-
- Pascual M, Theruvath T, Kawai T, Tolkoff‐Rubin N, Cosimi AB. Strategies to improve long‐term outcomes after renal transplantation. New England Journal of Medicine 2002;346(8):580‐90. - PubMed
Pittler 2000
-
- Pittler MH, Abbot NC, Harkness EF, Ernst E. Location bias in controlled clinical trials of complementary/alternative therapies. Journal of Clinical Epidemiology 2000;53(5):485‐9. [MEDLINE: ] - PubMed
Ramos 2009
-
- Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK‐associated nephropathy: state of affairs. Transplantation 2009;87(5):621‐30. [MEDLINE: ] - PubMed
Ridker 2006
-
- Ridker PM, Torres J. Reported outcomes in major cardiovascular clinical trials funded by for‐profit and not‐for‐profit organizations: 2000‐2005. [Erratum appears in JAMA. 2006 Jun 21;295(23):2726]. JAMA 2006;295(19):2270‐4. [MEDLINE: ] - PubMed
Salvadori 2004
-
- Salvadori M, Holzer H, Mattos A, Sollinger H, Arns W, Oppenheimer F, et al. Enteric‐coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. American Journal of Transplantation 2004;4(2):231‐6. [MEDLINE: ] - PubMed
Schmid 2004
-
- Schmid CH, Stark PC, Berlin JA, Landais P, Lau J. Meta‐regression detected associations between heterogeneous treatment effects and study‐level, but not patient‐level, factors. Journal of Clinical Epidemiology 2004;57(7):683‐97. [MEDLINE: ] - PubMed
Schold 2009
-
- Schold JD, Kaplan B. AZA/tacrolimus is associated with similar outcomes as MMF/tacrolimus among renal transplant recipients. American Journal of Transplantation 2009;9(9):2067‐74. [MEDLINE: ] - PubMed
Sifontis 2006
-
- Sifontis NM, Coscia LA, Constantinescu S, Lavelanet AF, Moritz MJ, Armenti VT. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006;82(12):1698‐702. [MEDLINE: ] - PubMed
Snyder 2009
-
- Snyder JJ, Israni AK, Peng Y, Zhang L, Simon TA, Kasiske BL. Rates of first infection following kidney transplant in the United States. Kidney International 2009;75(3):317‐26. [MEDLINE: ] - PubMed
Srinivas 2005
-
- Srinivas TR, Kaplan B, Schold JD, Meier‐Kriesche HU. The impact of mycophenolate mofetil on long‐term outcomes in kidney transplantation. Transplantation 2005;80(2 Suppl):S211‐20. [MEDLINE: ] - PubMed
Staatz 2007
-
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clinical Pharmacokinetics 2007;46(1):13‐58. [MEDLINE: ] - PubMed
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [MEDLINE: ] - PubMed
Tantravahi 2007
-
- Tantravahi J, Womer KL, Kaplan B. Why hasn't eliminating acute rejection improved graft survival?. Annual Review of Medicine 2007;58:369‐85. [MEDLINE: ] - PubMed
Terrin 2005
-
- Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. Journal of Clinical Epidemiology 2005;58(9):894‐901. [MEDLINE: ] - PubMed
Wagner 2009
Wallace 2009
Wang 2004a
-
- Wang K, Zhang H, Li Y, Wei Q, Li H, Yang Y, et al. Efficacy of mycophenolate mofetil versus azathioprine after renal transplantation: a systematic review. Transplantation Proceedings 2004;36:2071‐2. [MEDLINE: ] - PubMed
Wang 2004b
-
- Wang K, Zhang H, Li Y, Wei Q, Li H, Yang Y, et al. Safety of mycophenolate mofetil versus azathioprine in renal transplantation: a systematic review. Transplantation Proceedings 2004;36(7):2068‐70. [MEDLINE: ] - PubMed
Wang 2005
-
- Wang KJ, Zhang HT, Li YP, Lu YP, Wei Q, Li H, et al. Safety of mycophenolate mofetil versus azathioprin in renal transplantation: a systematic review. Chinese Journal of Evidence‐Based Medicine 2005;5(5):365‐74. [EMBASE: 2005250232]
Webster 2005
Webster 2006
Wimmer 2007
-
- Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, et al. The janus face of immunosuppression ‐ de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney International 2007;71(12):1271‐8. [MEDLINE: ] - PubMed
Zhang 2004
-
- Zhang H, Wang K, Li Y, Gao L, Liu J, Cai Y. Systematic review of randomized controlled trials about comparison mycophenolate mofetil and azathioprine after renal transplantation. Chinese Journal of Evidence‐Based Medicine 2004;4(2):79‐91.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

