The MARINA Risk Assessment Strategy: A Flexible Strategy for Efficient Information Collection and Risk Assessment of Nanomaterials
- PMID: 26633430
- PMCID: PMC4690897
- DOI: 10.3390/ijerph121214961
The MARINA Risk Assessment Strategy: A Flexible Strategy for Efficient Information Collection and Risk Assessment of Nanomaterials
Abstract
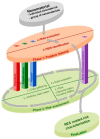
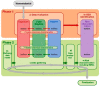
An engineered nanomaterial (ENM) may actually consist of a population of primary particles, aggregates and agglomerates of various sizes. Furthermore, their physico-chemical characteristics may change during the various life-cycle stages. It will probably not be feasible to test all varieties of all ENMs for possible health and environmental risks. There is therefore a need to further develop the approaches for risk assessment of ENMs. Within the EU FP7 project Managing Risks of Nanoparticles (MARINA) a two-phase risk assessment strategy has been developed. In Phase 1 (Problem framing) a base set of information is considered, relevant exposure scenarios (RESs) are identified and the scope for Phase 2 (Risk assessment) is established. The relevance of an RES is indicated by information on exposure, fate/kinetics and/or hazard; these three domains are included as separate pillars that contain specific tools. Phase 2 consists of an iterative process of risk characterization, identification of data needs and integrated collection and evaluation of data on the three domains, until sufficient information is obtained to conclude on possible risks in a RES. Only data are generated that are considered to be needed for the purpose of risk assessment. A fourth pillar, risk characterization, is defined and it contains risk assessment tools. This strategy describes a flexible and efficient approach for data collection and risk assessment which is essential to ensure safety of ENMs. Further developments are needed to provide guidance and make the MARINA Risk Assessment Strategy operational. Case studies will be needed to refine the strategy.
Keywords: exposure-driven; nanomaterials; problem framing; risk assessment strategy.
Figures
References
-
- Oomen A.G., Bos P.M., Fernandes T.F., Hund-Rinke K., Boraschi D., Byrne H.J., Aschberger K., Gottardo S., von der Kammer F., Kuhnel D., et al. Concern-driven integrated approaches to nanomaterial testing and assessment—Report of the nanosafety cluster working group 10. Nanotoxicology. 2014;8:334–348. doi: 10.3109/17435390.2013.802387. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

