VGLUTs and Glutamate Synthesis-Focus on DRG Neurons and Pain
- PMID: 26633536
- PMCID: PMC4693284
- DOI: 10.3390/biom5043416
VGLUTs and Glutamate Synthesis-Focus on DRG Neurons and Pain
Abstract
The amino acid glutamate is the principal excitatory transmitter in the nervous system, including in sensory neurons that convey pain sensation from the periphery to the brain. It is now well established that a family of membrane proteins, termed vesicular glutamate transporters (VGLUTs), serve a critical function in these neurons: they incorporate glutamate into synaptic vesicles. VGLUTs have a central role both under normal neurotransmission and pathological conditions, such as neuropathic or inflammatory pain. In the present short review, we will address VGLUTs in the context of primary afferent neurons. We will focus on the role of VGLUTs in pain triggered by noxious stimuli, peripheral nerve injury, and tissue inflammation, as mostly explored in transgenic mice. The possible interplay between glutamate biosynthesis and VGLUT-dependent packaging in synaptic vesicles, and its potential impact in various pain states will be presented.
Keywords: DRG; axotomy; glutamate; neuropathy; neuropeptides; pain; peripheral nerves; sensory neurons; vesicular glutamate transporter; visceral organs.
Figures

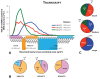
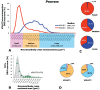
References
-
- Bennett G.J. Animal Models of Pain. In: Kruger L., editor. Methods in Pain Research. CRC Press; Boca Raton, FL, USA: 2001. pp. 67–92.
-
- Mogil J.S., Wilson S.G., Wan Y. Assessing nociception in murine subjects. In: Kruger L., editor. Methods in Pain Research. CRC Press; Boca Ratón, FL, USA: 2001. pp. 11–40.
-
- Wang J.J., Ho S.T., Hu O.Y., Chu K.M. An innovative cold tail-flick test: The cold ethanol tail-flick test. Anesth. Analg. 1995;80:102–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

