FAT4 functions as a tumour suppressor in gastric cancer by modulating Wnt/β-catenin signalling
- PMID: 26633557
- PMCID: PMC4701992
- DOI: 10.1038/bjc.2015.367
FAT4 functions as a tumour suppressor in gastric cancer by modulating Wnt/β-catenin signalling
Abstract
Background: FAT4, a cadherin-related protein, was shown to function as a tumour suppressor; however, its role in human gastric cancer remains largely unknown. Here, we investigated the role of FAT4 in gastric cancer and examined the underlying molecular mechanisms.
Methods: The expression of FAT4 was evaluated by immunohistochemistry, western blotting, and qRT-PCR in relation to the clinicopathological characteristics of gastric cancer patients. The effects of FAT4 silencing on cell proliferation, migration, and invasion were assessed by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) assay, and migration and invasion assays in gastric cancer cell lines in vitro and in a mouse xenograft model in vivo.
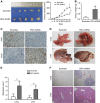
Results: Downregulation of FAT4 expression in gastric cancer tissues compared with adjacent normal tissues was correlated with lymph-node metastasis and poor survival. Knockdown of FAT4 promoted the growth and invasion of gastric cancer cells via the activation of Wnt/β-catenin signalling, and induced epithelial-to-mesenchymal transition (EMT) in gastric cancer cells, as demonstrated by the upregulation and downregulation of mesenchymal and epithelial markers. Silencing of FAT4 promoted tumour growth and metastasis in a gastric cancer xenograft model in vivo.
Conclusions: FAT4 has a tumour suppressor role mediated by the modulation of Wnt/β-catenin signalling, providing potential novel targets for the treatment of gastric cancer.
Figures





References
-
- Braun GS, Kretzler M, Heider T, Floege J, Holzman LB, Kriz W, Moeller MJ (2007) Differentially spliced isoforms of FAT1 are asymmetrically distributed within migrating cells. J Biol Chem 282: 22823–22833. - PubMed
-
- Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E (2009) Gastric cancer. Crit Rev Oncol Hematol 71: 127–164. - PubMed
-
- Clements WM, Wang J, Sarniak A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM (2002) β-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 62: 3503–3506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

