Primordial Follicle Transplantation within Designer Biomaterial Grafts Produce Live Births in a Mouse Infertility Model
- PMID: 26633657
- PMCID: PMC4668556
- DOI: 10.1038/srep17709
Primordial Follicle Transplantation within Designer Biomaterial Grafts Produce Live Births in a Mouse Infertility Model
Abstract
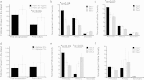
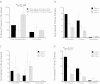
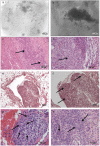
The gonadotoxic effects of chemotherapy and radiation may result in premature ovarian failure in premenopausal oncology patients. Although autotransplantation of ovarian tissue has led to successful live births, reintroduction of latent malignant cells inducing relapse is a significant concern. In this report, we investigated the design of biomaterial grafts for transplantation of isolated ovarian follicles as a means to preserve fertility. Primordial and primary ovarian follicles from young female mice were extracted and encapsulated into biomaterials for subsequent transplantation into adult mice. Among the formulations tested, aggregated follicles encapsulated within fibrin had enhanced survival and integration with the host tissue following transplantation relative to the fibrin-alginate and fibrin-collagen composites. All mice transplanted with fibrin-encapsulated follicles resumed cycling, and live births were achieved only for follicles transplanted within VEGF-loaded fibrin beads. The extent to which these procedures reduce the presence of metastatic breast cancer cells among the isolated follicles was evaluated, with significantly reduced numbers of cancer cells present relative to intact ovaries. This ability to obtain live births by transplanting isolated primordial and primary follicles, while also reducing the risk of re-seeding disease relative to ovarian tissue transplantation, may ultimately provide a means to preserve fertility in premenopausal oncology patients.
Figures



References
-
- Shapira M., Raanani H., Cohen Y. & Meirow D. Fertility preservation in young females with hematological malignancies. Acta Haematol 132, 400–413 (2014). - PubMed
-
- Partridge A. H. et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 22, 4174–4183 (2004). - PubMed
-
- Loscalzo M. & Clark K. L. The psychosocial context of cancer-related infertility. Cancer Treat Res 138, 180–190 (2007). - PubMed
-
- Jung M. et al. The clinical outcome of chemotherapy-induced amenorrhea in premenopausal young patients with breast cancer with long-term follow-up. Ann Surg Oncol 17, 3259–3268 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

