The role of rapid diagnostics in managing Ebola epidemics
- PMID: 26633764
- PMCID: PMC4823022
- DOI: 10.1038/nature16041
The role of rapid diagnostics in managing Ebola epidemics
Abstract
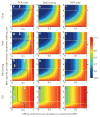
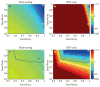
Ebola emerged in West Africa around December 2013 and swept through Guinea, Sierra Leone and Liberia, giving rise to 27,748 confirmed, probable and suspected cases reported by 29 July 2015. Case diagnoses during the epidemic have relied on polymerase chain reaction-based tests. Owing to limited laboratory capacity and local transport infrastructure, the delays from sample collection to test results being available have often been 2 days or more. Point-of-care rapid diagnostic tests offer the potential to substantially reduce these delays. We review Ebola rapid diagnostic tests approved by the World Health Organization and those currently in development. Such rapid diagnostic tests could allow early triaging of patients, thereby reducing the potential for nosocomial transmission. In addition, despite the lower test accuracy, rapid diagnostic test-based diagnosis may be beneficial in some contexts because of the reduced time spent by uninfected individuals in health-care settings where they may be at increased risk of infection; this also frees up hospital beds. We use mathematical modelling to explore the potential benefits of diagnostic testing strategies involving rapid diagnostic tests alone and in combination with polymerase chain reaction testing. Our analysis indicates that the use of rapid diagnostic tests with sensitivity and specificity comparable with those currently under development always enhances control, whether evaluated at a health-care-unit or population level. If such tests had been available throughout the recent epidemic, we estimate, for Sierra Leone, that their use in combination with confirmatory polymerase chain-reaction testing might have reduced the scale of the epidemic by over a third.
Conflict of interest statement
The authors declare no competing financial interests. Financial support for this publication has been provided by the Bill & Melinda Gates Foundation.
Figures


References
-
- World Health Organization. Laboratory Diagnosis of Ebola Virus Disease. WHO; 2014.
-
- World Health Organization. Urgently Needed: Rapid, Sensitive, Safe and Simple Ebola Diagnostic Tests. 2014 http://www.who.int/mediacentre/news/ebola/18-november-2014-diagnostics/en/
-
- Altona Diagnostics. Filovirus Screen RT-PCR Kit 1.0. Altona; 2014. RealStar®.
-
- Obelis SA, Shanghai ZJ Bio-Tech Co. Ltd. LifeRiver Ebola Virus (EBOV) Real Time RT-PCR Kit User Manual. Bio-Tech; 2012.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

