Elusive sources of variability of dystrophin rescue by exon skipping
- PMID: 26634117
- PMCID: PMC4667482
- DOI: 10.1186/s13395-015-0070-6
Elusive sources of variability of dystrophin rescue by exon skipping
Abstract
Background: Systemic delivery of anti-sense oligonucleotides to Duchenne muscular dystrophy (DMD) patients to induce de novo dystrophin protein expression in muscle (exon skipping) is a promising therapy. Treatment with Phosphorodiamidate morpholino oligomers (PMO) lead to shorter de novo dystrophin protein in both animal models and DMD boys who otherwise lack dystrophin; however, restoration of dystrophin has been observed to be highly variable. Understanding the factors causing highly variable induction of dystrophin expression in pre-clinical models would likely lead to more effective means of exon skipping in both pre-clinical studies and human clinical trials.
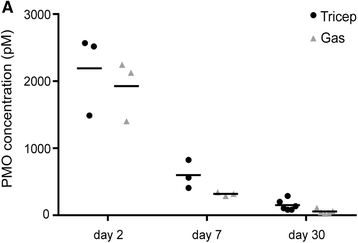
Methods: In the present study, we investigated possible factors that might lead to the variable success of exon skipping using morpholino drugs in the mdx mouse model. We tested whether specific muscle groups or fiber types showed better success than others and also correlated residual PMO concentration in muscle with the amount of de novo dystrophin protein 1 month after a single high-dose morpholino injection (800 mg/kg). We compared the results from six muscle groups using three different methods of dystrophin quantification: immunostaining, immunoblotting, and mass spectrometry assays.
Results: The triceps muscle showed the greatest degree of rescue (average 38±28 % by immunostaining). All three dystrophin detection methods were generally concordant for all muscles. We show that dystrophin rescue occurs in a sporadic patchy pattern with high geographic variability across muscle sections. We did not find a correlation between residual morpholino drug in muscle tissue and the degree of dystrophin expression.
Conclusions: While we found some evidence of muscle group enhancement and successful rescue, our data also suggest that other yet-undefined factors may underlie the observed variability in the success of exon skipping. Our study highlights the challenges associated with quantifying dystrophin in clinical trials where a single small muscle biopsy is taken from a DMD patient.
Keywords: Duchenne muscular dystrophy; Dystrophin; Exon skipping; Variability; mdx-23.
Figures





References
-
- Hoffman EP. Genotype/phenotype correlations in Duchenne/Becker dystrophy. Mol Cell Biol Hum Dis Serv. 1993;3:12–36. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

