Muscle networks: Connectivity analysis of EMG activity during postural control
- PMID: 26634293
- PMCID: PMC4669476
- DOI: 10.1038/srep17830
Muscle networks: Connectivity analysis of EMG activity during postural control
Abstract
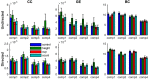
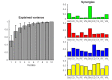
Understanding the mechanisms that reduce the many degrees of freedom in the musculoskeletal system remains an outstanding challenge. Muscle synergies reduce the dimensionality and hence simplify the control problem. How this is achieved is not yet known. Here we use network theory to assess the coordination between multiple muscles and to elucidate the neural implementation of muscle synergies. We performed connectivity analysis of surface EMG from ten leg muscles to extract the muscle networks while human participants were standing upright in four different conditions. We observed widespread connectivity between muscles at multiple distinct frequency bands. The network topology differed significantly between frequencies and between conditions. These findings demonstrate how muscle networks can be used to investigate the neural circuitry of motor coordination. The presence of disparate muscle networks across frequencies suggests that the neuromuscular system is organized into a multiplex network allowing for parallel and hierarchical control structures.
Figures
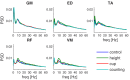


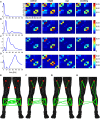
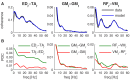
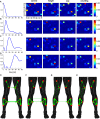
References
-
- Buzsaki G. & Draguhn A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004). - PubMed
-
- Engel A. K., Fries P. & Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2, 704–716 (2001). - PubMed
-
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9, 474–480 (2005). - PubMed
-
- Varela F., Lachaux J. P., Rodriguez E. & Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2, 229–239 (2001). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

