Vinculin controls talin engagement with the actomyosin machinery
- PMID: 26634421
- PMCID: PMC4686655
- DOI: 10.1038/ncomms10038
Vinculin controls talin engagement with the actomyosin machinery
Abstract
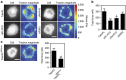
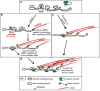
The link between extracellular-matrix-bound integrins and intracellular F-actin is essential for cell spreading and migration. Here, we demonstrate how the actin-binding proteins talin and vinculin cooperate to provide this link. By expressing structure-based talin mutants in talin null cells, we show that while the C-terminal actin-binding site (ABS3) in talin is required for adhesion complex assembly, the central ABS2 is essential for focal adhesion (FA) maturation. Thus, although ABS2 mutants support cell spreading, the cells lack FAs, fail to polarize and exert reduced force on the surrounding matrix. ABS2 is inhibited by the preceding mechanosensitive vinculin-binding R3 domain, and deletion of R2R3 or expression of constitutively active vinculin generates stable force-independent FAs, although cell polarity is compromised. Our data suggest a model whereby force acting on integrin-talin complexes via ABS3 promotes R3 unfolding and vinculin binding, activating ABS2 and locking talin into an actin-binding configuration that stabilizes FAs.
Figures






References
-
- Wehrle-Haller B. Assembly and disassembly of cell matrix adhesions. Curr. Opin. Cell. Biol. 24, 569–581 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

