Ranibizumab for the treatment of wet AMD: a summary of real-world studies
- PMID: 26634711
- PMCID: PMC4763117
- DOI: 10.1038/eye.2015.217
Ranibizumab for the treatment of wet AMD: a summary of real-world studies
Erratum in
-
Ranibizumab for the treatment of wet AMD: a summary of real-world studies.Eye (Lond). 2016 Nov;30(11):1526. doi: 10.1038/eye.2016.202. Epub 2016 Sep 9. Eye (Lond). 2016. PMID: 27612184 Free PMC article. No abstract available.
Abstract
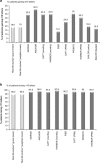
Data from real-world studies of ranibizumab in neovascular (wet) age-related macular degeneration suggest that outcomes in clinical practice fail to match those seen in clinical trials. These real-world studies follow treatment regimens that differ from the fixed dosing used in the pivotal clinical trial programme. To better understand the effectiveness of ranibizumab in clinical practice, we conducted a comprehensive evaluation of 12-month outcomes reported in peer-reviewed 'real-world' publications. Key measures included in our analysis were mean change in visual acuity (VA) and the proportion of patients gaining ≥15 letters or losing ≤15 letters. Twenty studies were eligible for inclusion in our study, with 18 358 eyes having sufficient data for analysis of 12-month outcomes. Mean baseline VA ranged from 48.8 to 61.6 Early Treatment Diabetic Retinopathy Study letters. Mean change in VA was between -2.0 and +5.5 letters, with a grand mean of +2.9±3.2, and a weighted mean (adjusted for the number of eyes in the study) of +1.95. Eleven studies reported that 19±7.5 (mean value) of patients gained ≥15 letters, while in 12 studies the mean percentage of patient losing ≤15 letters was 89±6.5%. Our comprehensive analysis of real-world ranibizumab study data confirm that patient outcomes are considerably poorer than those reported in randomised control trials of both fixed and pro re nata regimens.
Conflict of interest statement
VC is a consultant for Allergan, Bayer, Novartis, Quantel Medical, Pfenex.
Figures


References
-
- 2Kawasaki R, Yasuda M, Song SJ, Chen SJ, Jonas JB, Wang JJ et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology 2010; 117(5): 921–927. - PubMed
-
- 3Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab vs verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355(14): 1432–1444. - PubMed
-
- 4Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355(14): 1419–1431. - PubMed
-
- 5Blick SK, Keating GM, Wagstaff AJ. Ranibizumab. Drugs 2007; 67(8): 1199–1206 discussion 1207-1199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

