Mitigating the Effects of Nonadherence in Clinical Trials
- PMID: 26634893
- PMCID: PMC5066799
- DOI: 10.1002/jcph.689
Mitigating the Effects of Nonadherence in Clinical Trials
Abstract
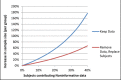
Accounting for subject nonadherence and eliminating inappropriate subjects in clinical trials are critical elements of a successful study. Nonadherence can increase variance, lower study power, and reduce the magnitude of treatment effects. Inappropriate subjects (including those who do not have the illness under study, fail to report exclusionary conditions, falsely report medication adherence, or participate in concurrent trials) confound safety and efficacy signals. This paper, a product of the International Society for CNS Clinical Trial Methodology (ISCTM) Working Group on Nonadherence in Clinical Trials, explores and models nonadherence in clinical trials and puts forth specific recommendations to identify and mitigate its negative effects. These include statistical analyses of nonadherence data, novel protocol design, and the use of biomarkers, subject registries, and/or medication adherence technologies.
Keywords: adherence; clinical trials; duplicate subjects; nonadherence; professional subjects.
© 2016, The American College of Clinical Pharmacology.
Figures



References
-
- World Health Organization . World Health Report 2003: Shaping the Future. Geneva, Switzerland: World Health Organization; 2003:17–19.
-
- National Institute for Health and Care Excellence. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. NICE clinical guideline 76. Available at www. nice.org.uk/guidance/cg76. Accessed October 2, 2015. - PubMed
-
- Osterberg L, Blaschke T. Drug therapy: adherence to mediation. N Engl J Med. 2005;353:487–497. - PubMed
-
- Gossec L, Tuback F, Dougados M, Ravaud P. Reporting of adherence to medication in recent randomized controlled trials of 6 chronic diseases: a systematic literature review. Am J Med Sci. 2007;334(4):248–254. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

