Prospective long-term minimal residual disease monitoring using RQ-PCR in RUNX1-RUNX1T1-positive acute myeloid leukemia: results of the French CBF-2006 trial
- PMID: 26635039
- PMCID: PMC4815724
- DOI: 10.3324/haematol.2015.131946
Prospective long-term minimal residual disease monitoring using RQ-PCR in RUNX1-RUNX1T1-positive acute myeloid leukemia: results of the French CBF-2006 trial
Abstract
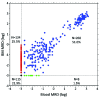
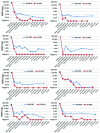
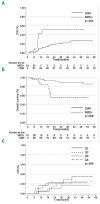
In t(8;21)(q22;q22) acute myeloid leukemia, the prognostic value of early minimal residual disease assessed with real-time quantitative polymerase chain reaction is the most important prognostic factor, but how long-term minimal residual disease monitoring may contribute to drive individual patient decisions remains poorly investigated. In the multicenter CBF-2006 study, a prospective monitoring of peripheral blood and bone marrow samples was performed every 3 months and every year, respectively, for 2 years following intensive chemotherapy in 94 patients in first complete remission. A complete molecular remission was defined as a (RUNX1-RUNX1T1/ABL1)×100 ≤ 0.001%. After the completion of consolidation therapy, a bone marrow complete molecular remission was observed in 30% of the patients, but was not predictive of subsequent relapse. Indeed, 8 patients (9%) presented a positive bone marrow minimal residual disease for up to 2 years of follow-up while still remaining in complete remission. Conversely, a peripheral blood complete molecular remission was statistically associated with a lower risk of relapse whatever the time-point considered after the completion of consolidation therapy. During the 2-year follow-up, the persistence of peripheral blood complete molecular remission was associated with a lower risk of relapse (4-year cumulative incidence, 8.2%), while molecular relapse confirmed on a subsequent peripheral blood sample predicted hematological relapse (4-year cumulative incidence, 86.9%) within a median time interval of 3.9 months. In t(8;21)(q22;q22) acute myeloid leukemia, minimal residual disease monitoring on peripheral blood every 3 months allows for the prediction of hematological relapse, and to identify patients who could potentially benefit from intervention therapy. (ClinicalTrials.gov ID #NCT00428558).
Copyright© Ferrata Storti Foundation.
Figures


References
-
- Yin JA, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120(14):2826–2835. - PubMed
-
- Jourdan E, Boissel N, Chevret S, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121(12):2213–2223. - PubMed
-
- Byrd JC, Weiss RB, Arthur DC, et al. Extramedullary leukemia adversely affects hematologic complete remission rate and overall survival in patients with t(8;21)(q22;q22): results from Cancer and Leukemia Group B 8461. J Clin Oncol. 1997;15(2):466–475. - PubMed
-
- Nguyen S, Leblanc T, Fenaux P, et al. A white blood cell index as the main prognostic factor in t(8;21) acute myeloid leukemia (AML): a survey of 161 cases from the French AML Intergroup. Blood. 2002;99(10):3517–3523. - PubMed
-
- Baer MR, Stewart CC, Lawrence D, et al. Expression of the neural cell adhesion molecule CD56 is associated with short remission duration and survival in acute myeloid leukemia with t(8;21)(q22;q22). Blood. 1997;90(4):1643–1648. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

