Protein and Signaling Networks in Vertebrate Photoreceptor Cells
- PMID: 26635520
- PMCID: PMC4646965
- DOI: 10.3389/fnmol.2015.00067
Protein and Signaling Networks in Vertebrate Photoreceptor Cells
Abstract
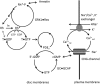
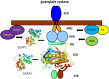
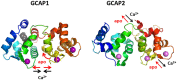
Vertebrate photoreceptor cells are exquisite light detectors operating under very dim and bright illumination. The photoexcitation and adaptation machinery in photoreceptor cells consists of protein complexes that can form highly ordered supramolecular structures and control the homeostasis and mutual dependence of the secondary messengers cyclic guanosine monophosphate (cGMP) and Ca(2+). The visual pigment in rod photoreceptors, the G protein-coupled receptor rhodopsin is organized in tracks of dimers thereby providing a signaling platform for the dynamic scaffolding of the G protein transducin. Illuminated rhodopsin is turned off by phosphorylation catalyzed by rhodopsin kinase (GRK1) under control of Ca(2+)-recoverin. The GRK1 protein complex partly assembles in lipid raft structures, where shutting off rhodopsin seems to be more effective. Re-synthesis of cGMP is another crucial step in the recovery of the photoresponse after illumination. It is catalyzed by membrane bound sensory guanylate cyclases (GCs) and is regulated by specific neuronal Ca(2+)-sensor proteins called guanylate cyclase-activating proteins (GCAPs). At least one GC (ROS-GC1) was shown to be part of a multiprotein complex having strong interactions with the cytoskeleton and being controlled in a multimodal Ca(2+)-dependent fashion. The final target of the cGMP signaling cascade is a cyclic nucleotide-gated (CNG) channel that is a hetero-oligomeric protein located in the plasma membrane and interacting with accessory proteins in highly organized microdomains. We summarize results and interpretations of findings related to the inhomogeneous organization of signaling units in photoreceptor outer segments.
Keywords: cGMP; calcium-binding proteins; multi-protein complexes; phototransduction; second messenger signaling.
Figures


References
-
- Ames J. B., Ishima R., Tanaka T., Gordon J. I., Stryer L., Ikura M. (1997). Molecular mechanics of calcium-myristoyl switches. Nature 389, 198–202. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

