Dual Nitrergic/Cholinergic Control of Short-Term Plasticity of Corticostriatal Inputs to Striatal Projection Neurons
- PMID: 26635532
- PMCID: PMC4655244
- DOI: 10.3389/fncel.2015.00453
Dual Nitrergic/Cholinergic Control of Short-Term Plasticity of Corticostriatal Inputs to Striatal Projection Neurons
Abstract
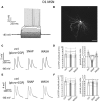
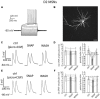
The ability of nitric oxide and acetylcholine to modulate the short-term plasticity of corticostriatal inputs was investigated using current-clamp recordings in BAC mouse brain slices. Glutamatergic responses were evoked by stimulation of corpus callosum in D1 and D2 dopamine receptor-expressing medium spiny neurons (D1-MSNs and D2-MSN, respectively). Paired-pulse stimulation (50 ms intervals) evoked depressing or facilitating responses in subgroups of both D1-MSNs and D2 MSNs. In both neuronal types, glutamatergic responses of cells that displayed paired-pulse depression were not significantly affected by the nitric oxide donor S-nitroso-N-acetylpenicillamine (SNAP; 100 μM). Conversely, in D1-MSNs and D2-MSNs that displayed paired-pulse facilitation, SNAP did not affect the first evoked response, but significantly reduced the amplitude of the second evoked EPSP, converting paired-pulse facilitation into paired-pulse depression. SNAP also strongly excited cholinergic interneurons and increased their cortical glutamatergic responses acting through a presynaptic mechanism. The effects of SNAP on glutamatergic response of D1-MSNs and D2-MSN were mediated by acetylcholine. The broad-spectrum muscarinic receptor antagonist atropine (25 μM) did not affect paired-pulse ratios and did not prevent the effects of SNAP. Conversely, the broad-spectrum nicotinic receptor antagonist tubocurarine (10 μM) fully mimicked and occluded the effects of SNAP. We concluded that phasic acetylcholine release mediates feedforward facilitation in MSNs through activation of nicotinic receptors on glutamatergic terminals and that nitric oxide, while increasing cholinergic interneurons' firing, functionally impairs their ability to modulate glutamatergic inputs of MSNs. These results show that nitrergic and cholinergic transmission control the short-term plasticity of glutamatergic inputs in the striatum and reveal a novel cellular mechanism underlying paired-pulse facilitation in this area.
Keywords: cholinergic interneuron; microcircuit; nitrergic interneuron; nitric oxide; striatum.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

