The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease
- PMID: 26635606
- PMCID: PMC4653301
- DOI: 10.3389/fphar.2015.00276
The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease
Abstract
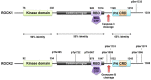
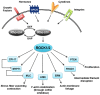
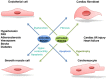
Rho-associated kinases ROCK1 and ROCK2 are serine/threonine kinases that are downstream targets of the small GTPases RhoA, RhoB, and RhoC. ROCKs are involved in diverse cellular activities including actin cytoskeleton organization, cell adhesion and motility, proliferation and apoptosis, remodeling of the extracellular matrix and smooth muscle cell contraction. The role of ROCK1 and ROCK2 has long been considered to be similar; however, it is now clear that they do not always have the same functions. Moreover, depending on their subcellular localization, activation, and other environmental factors, ROCK signaling can have different effects on cellular function. With respect to the heart, findings in isoform-specific knockout mice argue for a role of ROCK1 and ROCK2 in the pathogenesis of cardiac fibrosis and cardiac hypertrophy, respectively. Increased ROCK activity could play a pivotal role in processes leading to cardiovascular diseases such as hypertension, pulmonary hypertension, angina pectoris, vasospastic angina, heart failure, and stroke, and thus ROCK activity is a potential new biomarker for heart disease. Pharmacological ROCK inhibition reduces the enhanced ROCK activity in patients, accompanied with a measurable improvement in medical condition. In this review, we focus on recent findings regarding ROCK signaling in the pathogenesis of cardiovascular disease, with a special focus on differences between ROCK1 and ROCK2 function.
Keywords: ROCK; ROCK signaling; Rho-kinase; cardiovascular disease; fibrosis; heart; hypertrophy; inhibitor.
Figures
References
-
- Adyshev D. M., Moldobaeva N. K., Elangovan V. R., Garcia J. G., Dudek S. M. (2011). Differential involvement of ezrin/radixin/moesin proteins in sphingosine 1-phosphate-induced human pulmonary endothelial cell barrier enhancement. Cell. Signal. 23, 2086–2096. 10.1016/j.cellsig.2011.08.003 - DOI - PMC - PubMed
-
- Agrawal P. B., Joshi M., Savic T., Chen Z., Beggs A. H. (2012). Normal myofibrillar development followed by progressive sarcomeric disruption with actin accumulations in a mouse Cfl2 knockout demonstrates requirement of cofilin-2 for muscle maintenance. Hum. Mol. Genet. 21, 2341–2356. 10.1093/hmg/dds053 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

