Prefrontal Cortex and Social Cognition in Mouse and Man
- PMID: 26635701
- PMCID: PMC4659895
- DOI: 10.3389/fpsyg.2015.01805
Prefrontal Cortex and Social Cognition in Mouse and Man
Abstract
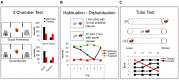
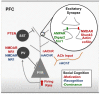
Social cognition is a complex process that requires the integration of a wide variety of behaviors, including salience, reward-seeking, motivation, knowledge of self and others, and flexibly adjusting behavior in social groups. Not surprisingly, social cognition represents a sensitive domain commonly disrupted in the pathology of a variety of psychiatric disorders including Autism Spectrum Disorder (ASD) and Schizophrenia (SCZ). Here, we discuss convergent research from animal models to human disease that implicates the prefrontal cortex (PFC) as a key regulator in social cognition, suggesting that disruptions in prefrontal microcircuitry play an essential role in the pathophysiology of psychiatric disorders with shared social deficits. We take a translational perspective of social cognition, and review three key behaviors that are essential to normal social processing in rodents and humans, including social motivation, social recognition, and dominance hierarchy. A shared prefrontal circuitry may underlie these behaviors. Social cognition deficits in animal models of neurodevelopmental disorders like ASD and SCZ have been linked to an altered balance of excitation and inhibition (E/I ratio) within the cortex generally, and PFC specifically. A clear picture of the mechanisms by which altered E/I ratio in the PFC might lead to disruptions of social cognition across a variety of behaviors is not well understood. Future studies should explore how disrupted developmental trajectory of prefrontal microcircuitry could lead to altered E/I balance and subsequent deficits in the social domain.
Keywords: autism; prefrontal cortex; schizophrenia; social behavior; social cognition.
Figures

References
-
- Akbarian S., Kim J. J., Potkin S. G., Hagman J. O., Tafazzoli A., Bunney W. E., Jr., et al. (1995). Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch. Gen. Psychiatry 52 258–266. 10.1001/archpsyc.1995.03950160008002 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

