Establishing a Role for Bacterial Cellulose in Environmental Interactions: Lessons Learned from Diverse Biofilm-Producing Proteobacteria
- PMID: 26635751
- PMCID: PMC4646962
- DOI: 10.3389/fmicb.2015.01282
Establishing a Role for Bacterial Cellulose in Environmental Interactions: Lessons Learned from Diverse Biofilm-Producing Proteobacteria
Abstract
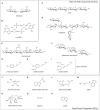
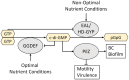
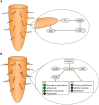
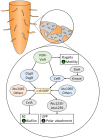
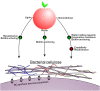
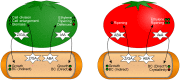
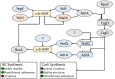
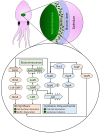
Bacterial cellulose (BC) serves as a molecular glue to facilitate intra- and inter-domain interactions in nature. Biosynthesis of BC-containing biofilms occurs in a variety of Proteobacteria that inhabit diverse ecological niches. The enzymatic and regulatory systems responsible for the polymerization, exportation, and regulation of BC are equally as diverse. Though the magnitude and environmental consequences of BC production are species-specific, the common role of BC-containing biofilms is to establish close contact with a preferred host to facilitate efficient host-bacteria interactions. Universally, BC aids in attachment, adherence, and subsequent colonization of a substrate. Bi-directional interactions influence host physiology, bacterial physiology, and regulation of BC biosynthesis, primarily through modulation of intracellular bis-(3'→5')-cyclic diguanylate (c-di-GMP) levels. Depending on the circumstance, BC producers exhibit a pathogenic or symbiotic relationship with plant, animal, or fungal hosts. Rhizobiaceae species colonize plant roots, Pseudomonadaceae inhabit the phyllosphere, Acetobacteriaceae associate with sugar-loving insects and inhabit the carposphere, Enterobacteriaceae use fresh produce as vehicles to infect animal hosts, and Vibrionaceae, particularly Aliivibrio fischeri, colonize the light organ of squid. This review will highlight the diversity of the biosynthesis and regulation of BC in nature by discussing various examples of Proteobacteria that use BC-containing biofilms to facilitate host-bacteria interactions. Through discussion of current data we will establish new directions for the elucidation of BC biosynthesis, its regulation and its ecophysiological roles.
Keywords: Komagataeibacter (Gluconacetobacter) xylinus; animal–bacteria interactions; bacterial cellulose; biofilms; c-di-GMP; ecophysiology; fungal–bacteria interactions; plant–bacteria interactions.
Figures

References
-
- Agravante J. U., Matsui T., Kitagawa H. (1990). Starch breakdown and changes in amylase activity during ripening of ethylene and ethanol treated bananas. Acta Hortic. 269 133–140. 10.17660/ActaHortic.1990.269.18 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

