Monocyte-Derived Dendritic Cells Are Essential for CD8(+) T Cell Activation and Antitumor Responses After Local Immunotherapy
- PMID: 26635798
- PMCID: PMC4655312
- DOI: 10.3389/fimmu.2015.00584
Monocyte-Derived Dendritic Cells Are Essential for CD8(+) T Cell Activation and Antitumor Responses After Local Immunotherapy
Abstract
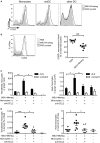
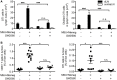
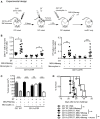
Tumors harbor several populations of dendritic cells (DCs) with the ability to prime tumor-specific T cells. However, these T cells mostly fail to differentiate into armed effectors and are unable to control tumor growth. We have previously shown that treatment with immunostimulatory agents at the tumor site can activate antitumor immune responses and is associated with the appearance of a population of monocyte-derived DCs (moDCs) in the tumor and tumor-draining lymph node (dLN). Here, we use depletion of DCs or monocytes and monocyte transfer to show that these moDCs are critical to the activation of antitumor immune responses. Treatment with the immunostimulatory agents monosodium urate crystals and Mycobacterium smegmatis induced the accumulation of monocytes in the dLN, their upregulation of CD11c and MHCII, and expression of iNOS, TNFα, and IL12p40. Blocking monocyte entry into the lymph node and tumor through neutralization of the chemokine CCL2 or inhibition of colony-stimulating factor-1 receptor signaling prevented the generation of moDCs, the infiltration of tumor-specific T cells into the tumor, and antitumor responses. In a reciprocal fashion, monocytes transferred into mice depleted of CD11c(+) cells were sufficient to rescue CD8(+) T cell priming in lymph node and delay tumor growth. Thus, monocytes exposed to the appropriate conditions become powerful activators of tumor-specific CD8(+) T cells and antitumor immunity.
Keywords: CSF1R; T cells; dendritic cells; monocyte-derived dendritic cells; mouse models; tumor immunotherapy.
Figures




References
-
- Stumbles PA, Himbeck R, Frelinger JA, Collins EJ, Lake RA, Robinson BW. Cutting edge: tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J Immunol (2004) 173:5923–8.10.4049/jimmunol.173.10.5923 - DOI - PubMed
-
- Movassagh M, Spatz A, Davoust J, Lebecque S, Romero P, Pittet M, et al. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res (2004) 64:2192–8.10.1158/0008-5472.CAN-03-2969 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

