Lactate promotes glutamine uptake and metabolism in oxidative cancer cells
- PMID: 26636483
- PMCID: PMC4825768
- DOI: 10.1080/15384101.2015.1120930
Lactate promotes glutamine uptake and metabolism in oxidative cancer cells
Abstract
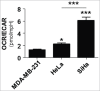
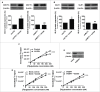
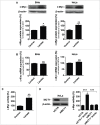
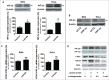
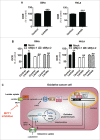
Oxygenated cancer cells have a high metabolic plasticity as they can use glucose, glutamine and lactate as main substrates to support their bioenergetic and biosynthetic activities. Metabolic optimization requires integration. While glycolysis and glutaminolysis can cooperate to support cellular proliferation, oxidative lactate metabolism opposes glycolysis in oxidative cancer cells engaged in a symbiotic relation with their hypoxic/glycolytic neighbors. However, little is known concerning the relationship between oxidative lactate metabolism and glutamine metabolism. Using SiHa and HeLa human cancer cells, this study reports that intracellular lactate signaling promotes glutamine uptake and metabolism in oxidative cancer cells. It depends on the uptake of extracellular lactate by monocarboxylate transporter 1 (MCT1). Lactate first stabilizes hypoxia-inducible factor-2α (HIF-2α), and HIF-2α then transactivates c-Myc in a pathway that mimics a response to hypoxia. Consequently, lactate-induced c-Myc activation triggers the expression of glutamine transporter ASCT2 and of glutaminase 1 (GLS1), resulting in improved glutamine uptake and catabolism. Elucidation of this metabolic dependence could be of therapeutic interest. First, inhibitors of lactate uptake targeting MCT1 are currently entering clinical trials. They have the potential to indirectly repress glutaminolysis. Second, in oxidative cancer cells, resistance to glutaminolysis inhibition could arise from compensation by oxidative lactate metabolism and increased lactate signaling.
Keywords: Cancer; Glutamine; Hypoxia-inducible factor (HIF); Tumor metabolism; c-Myc; glutaminolysis; hypoxia-inducible factor-2 (HIF-2); lactate signaling; monocarboxylate transporter 1 (MCT1).
Figures


References
-
- Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol 2011; 2:49; PMID:21904528; http://dx.doi.org/10.3389/fphar.2011.00049 - DOI - PMC - PubMed
-
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 2012; 21:297-308; PMID:22439925; http://dx.doi.org/10.1016/j.ccr.2012.02.014 - DOI - PMC - PubMed
-
- Wu R, Racker E. Regulatory mechanisms in carbohydrate metabolism. IV. Pasteur effect and Crabtree effect in ascites tumor cells. J Biol Chem 1959; 234:1036–41; PMID:13654314 - PubMed
-
- Kolobova E, Tuganova A, Boulatnikov I, Popov KM. Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites. Biochem J 2001; 358:69–77; PMID:11485553; http://dx.doi.org/10.1042/bj3580069 - DOI - PMC - PubMed
-
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006; 3:187–97; PMID:16517406; http://dx.doi.org/10.1016/j.cmet.2006.01.012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
