Tunable Keratin Hydrogels for Controlled Erosion and Growth Factor Delivery
- PMID: 26636618
- PMCID: PMC5565161
- DOI: 10.1021/acs.biomac.5b01328
Tunable Keratin Hydrogels for Controlled Erosion and Growth Factor Delivery
Abstract
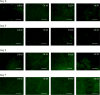
Tunable erosion of polymeric materials is an important aspect of tissue engineering for reasons that include cell infiltration, controlled release of therapeutic agents, and ultimately to tissue healing. In general, the biological response to proteinaceous polymeric hydrogels is favorable (e.g., minimal inflammatory response). However, unlike synthetic polymers, achieving tunable erosion with natural materials is a challenge. Keratins are a class of intermediate filament proteins that can be obtained from several sources, including human hair, and have gained increasing levels of use in tissue engineering applications. An important characteristic of keratin proteins is the presence of a large number of cysteine residues. Two classes of keratins with different chemical properties can be obtained by varying the extraction techniques: (1) keratose by oxidative extraction and (2) kerateine by reductive extraction. Cysteine residues of keratose are "capped" by sulfonic acid and are unable to form covalent cross-links upon hydration, whereas cysteine residues of kerateine remain as sulfhydryl groups and spontaneously form covalent disulfide cross-links. Here, we describe a straightforward approach to fabricate keratin hydrogels with tunable rates of erosion by mixing keratose and kerateine. SEM imaging and mechanical testing of freeze-dried materials showed similar pore diameters and compressive moduli, respectively, for each keratose-kerateine mixture formulation (∼1200 kPa for freeze-dried materials and ∼1.5 kPa for hydrogels). However, the elastic modulus (G') determined by rheology varied in proportion with the keratose-kerateine ratios, as did the rate of hydrogel erosion and the release rate of thiol from the hydrogels. The variation in keratose-kerateine ratios also led to tunable control over release rates of recombinant human insulin-like growth factor 1.
Figures


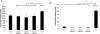

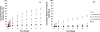

References
-
- Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharm. Res. 2003;20(8):1103–12. - PubMed
-
- Perez RA, Won JE, Knowles JC, Kim HW. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Del. Rev. 2013;65(4):471–96. - PubMed
-
- Kricheldorf HR, Berl M, Scharnagl N. Poly(Lactones) .9. Polymerization Mechanism of Metal Alkoxide Initiated Polymerizations of Lactide and Various Lactones. Macromolecules. 1988;21(2):286–293.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

