Gene Expression Switching of Receptor Subunits in Human Brain Development
- PMID: 26636753
- PMCID: PMC4670163
- DOI: 10.1371/journal.pcbi.1004559
Gene Expression Switching of Receptor Subunits in Human Brain Development
Abstract
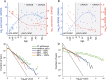
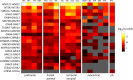
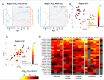
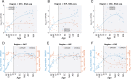
Synaptic receptors in the human brain consist of multiple protein subunits, many of which have multiple variants, coded by different genes, and are differentially expressed across brain regions and developmental stages. The brain can tune the electrophysiological properties of synapses to regulate plasticity and information processing by switching from one protein variant to another. Such condition-dependent variant switch during development has been demonstrated in several neurotransmitter systems including NMDA and GABA. Here we systematically detect pairs of receptor-subunit variants that switch during the lifetime of the human brain by analyzing postmortem expression data collected in a population of donors at various ages and brain regions measured using microarray and RNA-seq. To further detect variant pairs that co-vary across subjects, we present a method to quantify age-corrected expression correlation in face of strong temporal trends. This is achieved by computing the correlations in the residual expression beyond a cubic-spline model of the population temporal trend, and can be seen as a nonlinear version of partial correlations. Using these methods, we detect multiple new pairs of context dependent variants. For instance, we find a switch from GLRA2 to GLRA3 that differs from the known switch in the rat. We also detect an early switch from HTR1A to HTR5A whose trends are negatively correlated and find that their age-corrected expression is strongly positively correlated. Finally, we observe that GRIN2B switch to GRIN2A occurs mostly during embryonic development, presumably earlier than observed in rodents. These results provide a systematic map of developmental switching in the neurotransmitter systems of the human brain.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Aoki-Kinoshita KF, Kanehisa M. Gene annotation and pathway mapping in KEGG. Methods Mol Biol. 2007;396: 71–91. - PubMed
-
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28: 27–30. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=102409&tool=pm... - PMC - PubMed
-
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12: 4151–72. http://www.ncbi.nlm.nih.gov/pubmed/1331359 - PMC - PubMed
-
- Laurie DJ, Bartke I, Schoepfer R, Naujoks K, Seeburg PH. Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Mol Brain Res. 1997;51: 23–32. http://www.sciencedirect.com/science/article/pii/S0169328X97002064 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

