Lipoprotein (a): impact by ethnicity and environmental and medical conditions
- PMID: 26637279
- PMCID: PMC4918859
- DOI: 10.1194/jlr.R051904
Lipoprotein (a): impact by ethnicity and environmental and medical conditions
Abstract
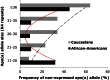
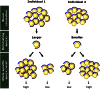
Levels of lipoprotein (a) [Lp(a)], a complex between an LDL-like lipid moiety containing one copy of apoB, and apo(a), a plasminogen-derived carbohydrate-rich hydrophilic protein, are primarily genetically regulated. Although stable intra-individually, Lp(a) levels have a skewed distribution inter-individually and are strongly impacted by a size polymorphism of the LPA gene, resulting in a variable number of kringle IV (KIV) units, a key motif of apo(a). The variation in KIV units is a strong predictor of plasma Lp(a) levels resulting in stable plasma levels across the lifespan. Studies have demonstrated pronounced differences across ethnicities with regard to Lp(a) levels and some of this difference, but not all of it, can be explained by genetic variations across ethnic groups. Increasing evidence suggests that age, sex, and hormonal impact may have a modest modulatory influence on Lp(a) levels. Among clinical conditions, Lp(a) levels are reported to be affected by kidney and liver diseases.
Keywords: apolipoprotein (a) size; demographic and clinical characteristics; diabetes; genetics; kidney and liver disease; populations.
Figures


References
-
- Dahlén G., Berg K., Gillnäs T., and Ericson C.. 1975. Lp(a) lipoprotein/pre-beta1-lipoprotein in Swedish middle-aged males and in patients with coronary heart disease. Clin. Genet. 7: 334–341. - PubMed
-
- Frick M. H., Dahlen G., Berg K., Valle M., and Hekali P.. 1978. Serum lipids in angiographically assessed coronary atherosclerosis. Chest. 73: 62–65. - PubMed
-
- Berg K., Dahlen G., and Borresen A. L.. 1979. Lp(a) phenotypes, other lipoprotein parameters, and a family history of coronary heart disease in middle-aged males. Clin. Genet. 16: 347–352. - PubMed
-
- Kostner G. M., Avogaro P., Cazzolato G., Marth E., Bittolo-Bon G., and Qunici G. B.. 1981. Lipoprotein Lp(a) and the risk for myocardial infarction. Atherosclerosis. 38: 51–61. - PubMed
-
- Guyton J. R., Dahlen G. H., Patsch W., Kautz J. A., and Gotto A. M. Jr. 1985. Relationship of plasma lipoprotein Lp(a) levels to race and to apolipoprotein B. Arteriosclerosis. 5: 265–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

