A Highly Conserved Residue of the HIV-1 gp120 Inner Domain Is Important for Antibody-Dependent Cellular Cytotoxicity Responses Mediated by Anti-cluster A Antibodies
- PMID: 26637462
- PMCID: PMC4733974
- DOI: 10.1128/JVI.02779-15
A Highly Conserved Residue of the HIV-1 gp120 Inner Domain Is Important for Antibody-Dependent Cellular Cytotoxicity Responses Mediated by Anti-cluster A Antibodies
Abstract
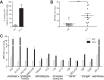
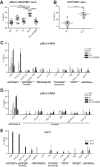
Previous studies have shown that sera from HIV-1-infected individuals contain antibodies able to mediate antibody-dependent cellular cytotoxicity (ADCC). These antibodies preferentially recognize envelope glycoprotein (Env) epitopes induced upon CD4 binding. Here, we show that a highly conserved tryptophan at position 69 of the gp120 inner domain is important for ADCC mediated by anti-cluster A antibodies and sera from HIV-1-infected individuals.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Veillette M, Coutu M, Richard J, Batraville LA, Dagher O, Bernard N, Tremblay C, Kaufmann DE, Roger M, Finzi A. 2015. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 89:545–551. doi:10.1128/JVI.02868-14. - DOI - PMC - PubMed
-
- Batraville LA, Richard J, Veillette M, Labbe AC, Alary M, Guedou F, Kaufmann DE, Poudrier J, Finzi A, Roger M. 2014. Anti-HIV-1 envelope immunoglobulin Gs in blood and cervicovaginal samples of Beninese commercial sex workers. AIDS Res Hum Retroviruses 30:1145–1149. doi:10.1089/aid.2014.0163. - DOI - PMC - PubMed
-
- Richard J, Veillette M, Brassard N, Iyer SS, Roger M, Martin L, Pazgier M, Schon A, Freire E, Routy JP, Smith AB III, Park J, Jones DM, Courter JR, Melillo BN, Kaufmann DE, Hahn BH, Permar SR, Haynes BF, Madani N, Sodroski JG, Finzi A. 2015. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 112:E2687–E2694. doi:10.1073/pnas.1506755112. - DOI - PMC - PubMed
-
- Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. 2014. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88:2633–2644. doi:10.1128/JVI.03230-13. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

