Blunted flow-mediated responses and diminished nitric oxide synthase expression in lymphatic thoracic ducts of a rat model of metabolic syndrome
- PMID: 26637560
- PMCID: PMC4796620
- DOI: 10.1152/ajpheart.00664.2015
Blunted flow-mediated responses and diminished nitric oxide synthase expression in lymphatic thoracic ducts of a rat model of metabolic syndrome
Abstract
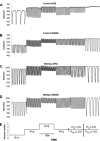
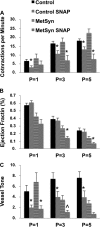
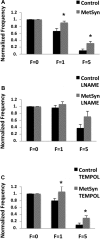
Shear-dependent inhibition of lymphatic thoracic duct (TD) contractility is principally mediated by nitric oxide (NO). Endothelial dysfunction and poor NO bioavailability are hallmarks of vasculature dysfunction in states of insulin resistance and metabolic syndrome (MetSyn). We tested the hypothesis that flow-dependent regulation of lymphatic contractility is impaired under conditions of MetSyn. We utilized a 7-wk high-fructose-fed male Sprague-Dawley rat model of MetSyn and determined the stretch- and flow-dependent contractile responses in an isobaric ex vivo TD preparation. TD diameters were tracked and contractile parameters were determined in response to different transmural pressures, imposed flow, exogenous NO stimulation by S-nitro-N-acetylpenicillamine (SNAP), and inhibition of NO synthase (NOS) by l-nitro-arginine methyl ester (l-NAME) and the reactive oxygen species (ROS) scavenging molecule 4-hydroxy-tempo (tempol). Expression of endothelial NO synthase (eNOS) in TD was determined using Western blot. Approximately 25% of the normal flow-mediated inhibition of contraction frequency was lost in TDs isolated from MetSyn rats despite a comparable SNAP response. Inhibition of NOS with l-NAME abolished the differences in the shear-dependent contraction frequency regulation between control and MetSyn TDs, whereas tempol did not restore the flow responses in MetSyn TDs. We found a significant reduction in eNOS expression in MetSyn TDs suggesting that diminished NO production is partially responsible for impaired flow response. Thus our data provide the first evidence that MetSyn conditions diminish eNOS expression in TD endothelium, thereby affecting the flow-mediated changes in TD lymphatic function.
Keywords: lymph flow; lymphatic endothelial cells; lymphatic vessel contraction; metabolic syndrome; nitric oxide synthase.
Copyright © 2016 the American Physiological Society.
Figures



References
-
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

