Metabolic Reprogramming of Stem Cell Epigenetics
- PMID: 26637942
- PMCID: PMC4672395
- DOI: 10.1016/j.stem.2015.11.012
Metabolic Reprogramming of Stem Cell Epigenetics
Abstract
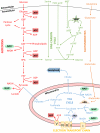
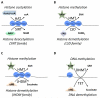
For many years, stem cell metabolism was viewed as a byproduct of cell fate status rather than an active regulatory mechanism; however, there is now a growing appreciation that metabolic pathways influence epigenetic changes associated with lineage commitment, specification, and self-renewal. Here we review how metabolites generated during glycolytic and oxidative processes are utilized in enzymatic reactions leading to epigenetic modifications and transcriptional regulation. We discuss how "metabolic reprogramming" contributes to global epigenetic changes in the context of naive and primed pluripotent states, somatic reprogramming, and hematopoietic and skeletal muscle tissue stem cells, and we discuss the implications for regenerative medicine.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
-
- Brinster RL, Troike DE. Requirements for blastocyst development in vitro. Journal of animal science. 1979;49(Suppl 2):26–34. - PubMed
-
- Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

