Pediatric gliomas as neurodevelopmental disorders
- PMID: 26638183
- PMCID: PMC4833573
- DOI: 10.1002/glia.22945
Pediatric gliomas as neurodevelopmental disorders
Abstract
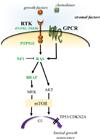
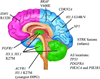
Brain tumors represent the most common solid tumor of childhood, with gliomas comprising the largest fraction of these cancers. Several features distinguish them from their adult counterparts, including their natural history, causative genetic mutations, and brain locations. These unique properties suggest that the cellular and molecular etiologies that underlie their development and maintenance might be different from those that govern adult gliomagenesis and growth. In this review, we discuss the genetic basis for pediatric low-grade and high-grade glioma in the context of developmental neurobiology, and highlight the differences between histologically-similar tumors arising in children and adults.
Keywords: DIPG; diffuse astrocytoma; glioblastoma; pediatric glioma; pilocytic astrocytoma.
© 2015 Wiley Periodicals, Inc.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

