Iron-overload injury and cardiomyopathy in acquired and genetic models is attenuated by resveratrol therapy
- PMID: 26638758
- PMCID: PMC4671148
- DOI: 10.1038/srep18132
Iron-overload injury and cardiomyopathy in acquired and genetic models is attenuated by resveratrol therapy
Abstract
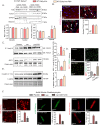
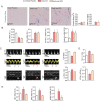
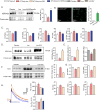
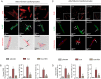
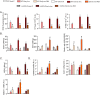
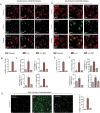
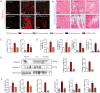
Iron-overload cardiomyopathy is a prevalent cause of heart failure on a world-wide basis and is a major cause of mortality and morbidity in patients with secondary iron-overload and genetic hemochromatosis. We investigated the therapeutic effects of resveratrol in acquired and genetic models of iron-overload cardiomyopathy. Murine iron-overload models showed cardiac iron-overload, increased oxidative stress, altered Ca(2+) homeostasis and myocardial fibrosis resulting in heart disease. Iron-overload increased nuclear and acetylated levels of FOXO1 with corresponding inverse changes in SIRT1 levels in the heart corrected by resveratrol therapy. Resveratrol, reduced the pathological remodeling and improved cardiac function in murine models of acquired and genetic iron-overload at varying stages of iron-overload. Echocardiography and hemodynamic analysis revealed a complete normalization of iron-overload mediated diastolic and systolic dysfunction in response to resveratrol therapy. Myocardial SERCA2a levels were reduced in iron-overloaded hearts and resveratrol therapy restored SERCA2a levels and corrected altered Ca(2+) homeostasis. Iron-mediated pro-oxidant and pro-fibrotic effects in human and murine cardiomyocytes and cardiofibroblasts were suppressed by resveratrol which correlated with reduction in iron-induced myocardial oxidative stress and myocardial fibrosis. Resveratrol represents a clinically and economically feasible therapeutic intervention to reduce the global burden from iron-overload cardiomyopathy at early and chronic stages of iron-overload.
Figures

References
-
- Barton J. C. & Bertoli L. F. Hemochromatosis: the genetic disorder of the twenty-first century. Nat. Med. 2, 394–395 (1996). - PubMed
-
- Andrews N. C. Disorders of iron metabolism. N. Engl. J. Med. 341, 1986–1995 (1999). - PubMed
-
- Pietrangelo A. Hereditary hemochromatosis–a new look at an old disease. N. Engl. J. Med. 350, 2383–2397 (2004). - PubMed
-
- Fleming R. E. & Ponka P. Iron overload in human disease. N. Engl. J. Med. 366, 348–359 (2012). - PubMed
-
- Murphy C. J. & Oudit G. Y. Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. J. Card. Fail. 16, 888–900 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

