Ceramides And Stress Signalling Intersect With Autophagic Defects In Neurodegenerative Drosophila blue cheese (bchs) Mutants
- PMID: 26639035
- PMCID: PMC4671070
- DOI: 10.1038/srep15926
Ceramides And Stress Signalling Intersect With Autophagic Defects In Neurodegenerative Drosophila blue cheese (bchs) Mutants
Abstract
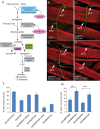
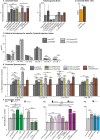
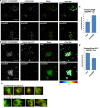
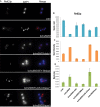
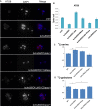
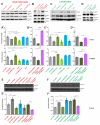
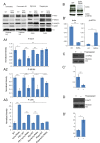
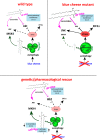
Sphingolipid metabolites are involved in the regulation of autophagy, a degradative recycling process that is required to prevent neuronal degeneration. Drosophila blue cheese mutants neurodegenerate due to perturbations in autophagic flux, and consequent accumulation of ubiquitinated aggregates. Here, we demonstrate that blue cheese mutant brains exhibit an elevation in total ceramide levels; surprisingly, however, degeneration is ameliorated when the pool of available ceramides is further increased, and exacerbated when ceramide levels are decreased by altering sphingolipid catabolism or blocking de novo synthesis. Exogenous ceramide is seen to accumulate in autophagosomes, which are fewer in number and show less efficient clearance in blue cheese mutant neurons. Sphingolipid metabolism is also shifted away from salvage toward de novo pathways, while pro-growth Akt and MAP pathways are down-regulated, and ER stress is increased. All these defects are reversed under genetic rescue conditions that increase ceramide generation from salvage pathways. This constellation of effects suggests a possible mechanism whereby the observed deficit in a potentially ceramide-releasing autophagic pathway impedes survival signaling and exacerbates neuronal death.
Figures
References
-
- Pettus B. J., Chalfant C. E. & Hannun Y. A. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 1585, 114–125, S1388198102003311 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

