A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study
- PMID: 26639181
- PMCID: PMC4832072
- DOI: 10.1038/leu.2015.313
A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study
Abstract
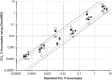
In chronic lymphocytic leukemia (CLL) the level of minimal residual disease (MRD) after therapy is an independent predictor of outcome. Given the increasing number of new agents being explored for CLL therapy, using MRD as a surrogate could greatly reduce the time necessary to assess their efficacy. In this European Research Initiative on CLL (ERIC) project we have identified and validated a flow-cytometric approach to reliably quantitate CLL cells to the level of 0.0010% (10(-5)). The assay comprises a core panel of six markers (i.e. CD19, CD20, CD5, CD43, CD79b and CD81) with a component specification independent of instrument and reagents, which can be locally re-validated using normal peripheral blood. This method is directly comparable to previous ERIC-designed assays and also provides a backbone for investigation of new markers. A parallel analysis of high-throughput sequencing using the ClonoSEQ assay showed good concordance with flow cytometry results at the 0.010% (10(-4)) level, the MRD threshold defined in the 2008 International Workshop on CLL guidelines, but it also provides good linearity to a detection limit of 1 in a million (10(-6)). The combination of both technologies would permit a highly sensitive approach to MRD detection while providing a reproducible and broadly accessible method to quantify residual disease and optimize treatment in CLL.
Conflict of interest statement
The type of relationship with the organizations are: ACR: Celgene, Honoraria; BD Biosciences, royalty payments (Intrasure reagent) and study reagents; Abbvie, Honoraria; Gilead, Consultancy and Honoraria; Biogen Idec, Consultancy; Roche, Honoraria; and GSK, Honoraria. PG: AbbVie, Honoraria; Adaptive Biotechnologies, Consultancy; Celgene, Consultancy; Gilead, Consultancy, Honoraria and research funds; GSK, research funds; Janssen, Consultancy and Honoraria; Pharmacyclics, Consultancy; and Roche, Research funds. All other co-authors report no conflicts of interest.
Figures




References
-
- Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–1174. - PubMed
-
- Montserrat E. Treatment of chronic lymphocytic leukemia: achieving minimal residual disease-negative status as a goal. J Clin Oncol 2005; 23: 2884–2885. - PubMed
-
- Research C for DE and News & Events—Public Workshop on Minimal Residual Disease (MRD) as a Surrogate Endpoint in Chronic Lymphocytic Leukemia (CLL). http://www.fda.gov/Drugs/NewsEvents/ucm340707.htm (accessed 2 February 2015).
-
- Guideline on the use of minimal residue disease as an endpoint in chronic lymphocytic leukaemia studies. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... (accessed 3 February 2015).
-
- Böttcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol 2012; 30: 980–988. - PubMed

