Self-directed exploration provides a Ncs1-dependent learning bonus
- PMID: 26639399
- PMCID: PMC4671055
- DOI: 10.1038/srep17697
Self-directed exploration provides a Ncs1-dependent learning bonus
Abstract
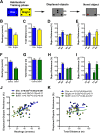
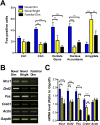
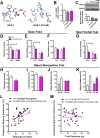
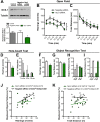
Understanding the mechanisms of memory formation is fundamental to establishing optimal educational practices and restoring cognitive function in brain disease. Here, we show for the first time in a non-primate species, that spatial learning receives a special bonus from self-directed exploration. In contrast, when exploration is escape-oriented, or when the full repertoire of exploratory behaviors is reduced, no learning bonus occurs. These findings permitted the first molecular and cellular examinations into the coupling of exploration to learning. We found elevated expression of neuronal calcium sensor 1 (Ncs1) and dopamine type-2 receptors upon self-directed exploration, in concert with increased neuronal activity in the hippocampal dentate gyrus and area CA3, as well as the nucleus accumbens. We probed further into the learning bonus by developing a point mutant mouse (Ncs1(P144S/P144S)) harboring a destabilized NCS-1 protein, and found this line lacked the equivalent self-directed exploration learning bonus. Acute knock-down of Ncs1 in the hippocampus also decoupled exploration from efficient learning. These results are potentially relevant for augmenting learning and memory in health and disease, and provide the basis for further molecular and circuit analyses in this direction.
Figures
References
-
- Berlyne D. E. Conflict, arousal, and curiosity. (McGraw-Hill, 1960).
-
- Montgomery K. C. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol 48, 254–260 (1955). - PubMed
-
- Zimbardo P. G. & Montgomery K. C. The relative strengths of consummatory responses in hunger, thirst, and exploratory drive. J Comp Physiol Psychol 50, 504–508 (1957). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

