An Efficient Procedure for Removal and Inactivation of Alpha-Synuclein Assemblies from Laboratory Materials
- PMID: 26639448
- PMCID: PMC4927840
- DOI: 10.3233/JPD-150691
An Efficient Procedure for Removal and Inactivation of Alpha-Synuclein Assemblies from Laboratory Materials
Abstract
Background: Preformed α-synuclein fibrils seed the aggregation of soluble α-synuclein in cultured cells and in vivo. This, and other findings, has kindled the idea that α-synuclein fibrils possess prion-like properties.
Objective: As α-synuclein fibrils should not be considered as innocuous, there is a need for decontamination and inactivation procedures for laboratory benches and non-disposable laboratory material.
Methods: We assessed the effectiveness of different procedures designed to disassemble α-synuclein fibrils and reduce their infectivity. We examined different commercially available detergents to remove α-synuclein assemblies adsorbed on materials that are not disposable and that are most found in laboratories (e.g. plastic, glass, aluminum or stainless steel surfaces).
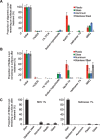
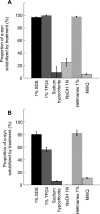
Results: We show that methods designed to decrease PrP prion infectivity neither effectively remove α-synuclein assemblies adsorbed to different materials commonly used in the laboratory nor disassemble the fibrillar form of the protein with efficiency. In contrast, both commercial detergents and SDS detached α-synuclein assemblies from contaminated surfaces and disassembled the fibrils.
Conclusions: We describe three cleaning procedures that effectively remove and disassemble α-synuclein seeds. The methods rely on the use of detergents that are compatible with most non-disposable tools in a laboratory. The procedures are easy to implement and significantly decrease any potential risks associated to handling α-synuclein assemblies.
Keywords: Alpha synuclein; Parkinson’s disease; cleaning procedures; detergent; fibrils; inactivation; removal.
Figures
References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, & Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature, 388, 839–840. - PubMed
-
- Gai WP, Power JH, Blumbergs PC, & Blessing WW (1998) Multiple-system atrophy: A new alpha-synuclein disease? Lancet, 352, 547–548. - PubMed
-
- Newell KL, Boyer P, Gomez-Tortosa E, Hobbs W, Hedley-Whyte ET, Vonsattel JP, & Hyman BT (1999) Alpha-synuclein immunoreactivity is present in axonal swellings in neuroal dystrophy and acute traumatic brain injury. J Neuropathol Exp Neurol, 58, 1263–1268. - PubMed
-
- Goldberg MS, & Lansbury PT Jr. (2000) Is there a cause-andeffect relationship between a-synuclein fibrilization and Parkinson’s disease? Nat Cell Biol, 2, E115–E119. - PubMed
-
- Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, & Fink AL (2002) Biophysical properties of the synucleins and their propensities to fibrillate: Inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem, 277, 11970–11978. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

