Update on pathogenesis and predictors of response of therapeutic strategies used in inflammatory bowel disease
- PMID: 26640330
- PMCID: PMC4658608
- DOI: 10.3748/wjg.v21.i44.12519
Update on pathogenesis and predictors of response of therapeutic strategies used in inflammatory bowel disease
Abstract
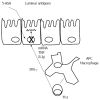
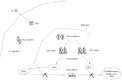
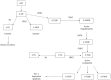
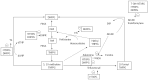
The search for biomarkers that characterize specific aspects of inflammatory bowel disease (IBD), has received substantial interest in the past years and is moving forward rapidly with the help of modern technologies. Nevertheless, there is a direct demand to identify adequate biomarkers for predicting and evaluating therapeutic response to different therapies. In this subset, pharmacogenetics deserves more attention as part of the endeavor to provide personalized medicine. The ultimate goal in this area is the adjustment of medication for a patient's specific genetic background and thereby to improve drug efficacy and safety rates. The aim of the following review is to utilize the latest knowledge on immunopathogenesis of IBD and update the findings on the field of Immunology and Genetics, to evaluate the response to the different therapies. In the present article, more than 400 publications were reviewed but finally 287 included based on design, reproducibility (or expectancy to be reproducible and translationable into humans) or already measured in humans. A few tests have shown clinical applicability. Other, i.e., genetic associations for the different therapies in IBD have not yet shown consistent or robust results. In the close future it is anticipated that this, cellular and genetic material, as well as the determination of biomarkers will be implemented in an integrated molecular diagnostic and prognostic approach to manage IBD patients.
Keywords: Biomarkers; Mode of action; Mucosal immunology; Pharmacology; Therapeutic drug monitoring.
Figures


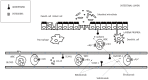
References
-
- Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. - PubMed
-
- Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–97. - PubMed
-
- Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhães JG, Yuan L, Soares F, Chea E, Le Bourhis L, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

