Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia
- PMID: 26641137
- PMCID: PMC4862586
- DOI: 10.1056/NEJMoa1509981
Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia
Abstract
Background: Irreversible inhibition of Bruton's tyrosine kinase (BTK) by ibrutinib represents an important therapeutic advance for the treatment of chronic lymphocytic leukemia (CLL). However, ibrutinib also irreversibly inhibits alternative kinase targets, which potentially compromises its therapeutic index. Acalabrutinib (ACP-196) is a more selective, irreversible BTK inhibitor that is specifically designed to improve on the safety and efficacy of first-generation BTK inhibitors.
Methods: In this uncontrolled, phase 1-2, multicenter study, we administered oral acalabrutinib to 61 patients who had relapsed CLL to assess the safety, efficacy, pharmacokinetics, and pharmacodynamics of acalabrutinib. Patients were treated with acalabrutinib at a dose of 100 to 400 mg once daily in the dose-escalation (phase 1) portion of the study and 100 mg twice daily in the expansion (phase 2) portion.
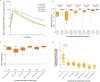
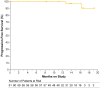
Results: The median age of the patients was 62 years, and patients had received a median of three previous therapies for CLL; 31% had chromosome 17p13.1 deletion, and 75% had unmutated immunoglobulin heavy-chain variable genes. No dose-limiting toxic effects occurred during the dose-escalation portion of the study. The most common adverse events observed were headache (in 43% of the patients), diarrhea (in 39%), and increased weight (in 26%). Most adverse events were of grade 1 or 2. At a median follow-up of 14.3 months, the overall response rate was 95%, including 85% with a partial response and 10% with a partial response with lymphocytosis; the remaining 5% of patients had stable disease. Among patients with chromosome 17p13.1 deletion, the overall response rate was 100%. No cases of Richter's transformation (CLL that has evolved into large-cell lymphoma) and only one case of CLL progression have occurred.
Conclusions: In this study, the selective BTK inhibitor acalabrutinib had promising safety and efficacy profiles in patients with relapsed CLL, including those with chromosome 17p13.1 deletion. (Funded by the Acerta Pharma and others; ClinicalTrials.gov number, NCT02029443.).
Figures

Comment in
-
Progress in Chronic Lymphocytic Leukemia with Targeted Therapy.N Engl J Med. 2016 Jan 28;374(4):386-8. doi: 10.1056/NEJMe1515235. N Engl J Med. 2016. PMID: 26816016 No abstract available.
-
New Agents to Treat Chronic Lymphocytic Leukemia.N Engl J Med. 2016 Jun 2;374(22):2185-6. doi: 10.1056/NEJMc1602674. N Engl J Med. 2016. PMID: 27248633 No abstract available.
References
-
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. The New England journal of medicine. 2014;370:1101–10. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
