Vitamin D Activation and Function in Human Corneal Epithelial Cells During TLR-Induced Inflammation
- PMID: 26641549
- PMCID: PMC4675205
- DOI: 10.1167/iovs.15-17768
Vitamin D Activation and Function in Human Corneal Epithelial Cells During TLR-Induced Inflammation
Abstract
Purpose: Vitamin D is recognized to be an important modulator of the immune system. In the eye, studies have shown that deficiencies and genetic differences in vitamin D-related genes have a significant impact on the development of various ocular diseases. Our current study examines the ability of human corneal epithelial cells (HCEC) to activate vitamin D and the effect of vitamin D treatment on antimicrobial peptide production and cytokine modulation during inflammation, with the ultimate goal of using vitamin D therapeutically for corneal inflammation.
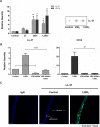
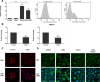
Methods: Human corneal epithelial cells were treated with 10-7M vitamin D3 (D3) or 25-hydroxyvitamin D3 (25D3) for 24 hours and 1,25-dihydroxyvitamin D3 (1,25D3) detected by immunoassay. Human cathelicidin (LL-37) expression was examined by RT-PCR, immunoblot, and immunostaining following 1,25D3 treatment and antimicrobial activity of 1,25D3-treated cells was determined. Cells were stimulated with TLR3 agonist polyinosinic-polycytidylic acid (Poly[I:C]) for 24 hours and cytokine levels measured by RT-PCR, ELISA, and Luminex. Immunostaining determined expression of vitamin D receptor (VDR) and retinoic acid inducible gene-1 receptor (RIG-1) as well as NF-κB nuclear translocation.
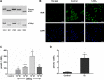
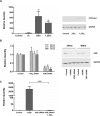
Results: When treated with inactive vitamin D metabolites, HCEC produced active 1,25D3, leading to enhanced expression of the antimicrobial peptide, LL-37, dependent on VDR. 1,25-D3 decreased the expression of proinflammatory cytokines (IL-1β, IL-6, TNFα, and CCL20) and MMP-9 induced by Poly(I:C) as well as pattern recognition receptor expression (TLR3, RIG-1, MDA5). However, early activation of NF-κB was not affected.
Conclusions: These studies demonstrate the protective ability of vitamin D to attenuate proinflammatory mediators while increasing antimicrobial peptides and antipseudomonas activity in corneal cells, and further our knowledge on the immunomodulatory functions of the hormone.
Figures


References
-
- Prosser DE,, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004; 29: 664–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

