Gene Delivery by Subconjunctival Injection of Adenovirus in Rats: A Study of Local Distribution, Transgene Duration and Safety
- PMID: 26642208
- PMCID: PMC4671571
- DOI: 10.1371/journal.pone.0143956
Gene Delivery by Subconjunctival Injection of Adenovirus in Rats: A Study of Local Distribution, Transgene Duration and Safety
Abstract
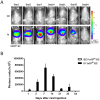
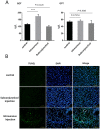
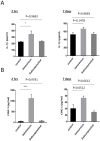
Subconjunctival injection is a minimally invasive route for gene delivery to ocular tissues, but has traditionally been limited to use in the cornea. The accurate ocular distribution of virus has not, however, been previously investigated. Adenovirus is an attractive gene vector as it can deliver large genes and allow for short-term gene expression, but how safe it is when delivered via subconjunctival injection remains to be established. We have characterized the bio-distribution and safety of subconjunctivally administered adenovirus in Brown Norway rats. The bio-distribution and transgene duration of adenovirus carrying luciferase gene (Ad-Luci) at various time intervals were evaluated via bioluminescence imaging after subconjunctival injection. Adenovirus carrying a reporter gene, β-galactosidase (Ad-LacZ) or hrGFP (Ad-hrGFP) was administered subconjunctivally and the viral distribution in various ocular tissues was assessed by histological analysis and quantitative PCR (qPCR). Hepatic damage was assessed by biochemical and immunohistological analysis with TUNEL stain. Systemic immunogenicity was assessed by measuring serum level of TNF-α via ELISA, 2 hours and 14 days after administration of adenovirus. Retinal function was examined by electroretinography. Subconjunctival injection of Ad-Luci induced luciferase expression in the injected eyes within 24 hours, for at least 64 days. Histological analysis showed adenovirus distributed across anterior and posterior ocular tissues. qPCR demonstrated different amounts of adenovirus in different ocular tissues, with the highest amounts closest to the injection site Unlike the intravenous route, subconjunctivally delivered adenovirus did not elicit any detectable hepatic injury or systemic immunogenicity. Retinal function was unaffected by adenovirus irrespective of administration route. In conclusion, an adenoviral vector administered subconjunctivally can infiltrate into different ocular tissues and lead to short-term ocular transgene expression, without causing hepatic injury and immune activation. Therefore, subconjunctivally administered adenovirus may be a promising gene delivery approach for managing anterior and posterior segment eye diseases requiring short-term therapy.
Conflict of interest statement
Figures


Similar articles
-
Characterization of adenovirus p21 gene transfer, biodistribution, and immune response after local ocular delivery in New Zealand white rabbits.Exp Eye Res. 2003 Sep;77(3):355-65. doi: 10.1016/s0014-4835(03)00122-2. Exp Eye Res. 2003. PMID: 12907168
-
Periocular triamcinolone enhances intraocular gene expression after delivery by adenovirus.Invest Ophthalmol Vis Sci. 2008 Jan;49(1):399-406. doi: 10.1167/iovs.07-0619. Invest Ophthalmol Vis Sci. 2008. PMID: 18172118
-
Long-term retinal transgene expression with FIV versus adenoviral vectors.Mol Vis. 2004 Apr 13;10:272-80. Mol Vis. 2004. PMID: 15094709
-
Adenoviral vectors for cardiovascular gene therapy applications: a clinical and industry perspective.J Mol Med (Berl). 2022 Jun;100(6):875-901. doi: 10.1007/s00109-022-02208-0. Epub 2022 May 24. J Mol Med (Berl). 2022. PMID: 35606652 Free PMC article. Review.
-
Adenovirus vectors for gene delivery.Curr Opin Biotechnol. 1999 Oct;10(5):440-7. doi: 10.1016/s0958-1669(99)00007-5. Curr Opin Biotechnol. 1999. PMID: 10508634 Review.
Cited by
-
Delta-like 1 homologue promotes tumorigenesis and epithelial-mesenchymal transition of ovarian high-grade serous carcinoma through activation of Notch signaling.Oncogene. 2019 Apr;38(17):3201-3215. doi: 10.1038/s41388-018-0658-5. Epub 2019 Jan 9. Oncogene. 2019. PMID: 30626939
-
Ocular Gene Therapy: An Overview of Viral Vectors, Immune Responses, and Future Directions.Yale J Biol Med. 2024 Dec 19;97(4):491-503. doi: 10.59249/HWID7537. eCollection 2024 Dec. Yale J Biol Med. 2024. PMID: 39703610 Free PMC article. Review.
-
Sustained and Efficient Delivery of Antivascular Endothelial Growth Factor by the Adeno-associated Virus for the Treatment of Corneal Neovascularization: An Outlook for Its Clinical Translation.J Ophthalmol. 2024 Sep 9;2024:5487973. doi: 10.1155/2024/5487973. eCollection 2024. J Ophthalmol. 2024. PMID: 39286553 Free PMC article. Review.
-
Viral-mediated Ntf3 overexpression disrupts innervation and hearing in nondeafened guinea pig cochleae.Mol Ther Methods Clin Dev. 2016 Aug 3;3:16052. doi: 10.1038/mtm.2016.52. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 27525291 Free PMC article.
-
Inhibition of miR-153 ameliorates ischemia/reperfusion-induced cardiomyocytes apoptosis by regulating Nrf2/HO-1 signaling in rats.Biomed Eng Online. 2020 Mar 6;19(1):15. doi: 10.1186/s12938-020-0759-6. Biomed Eng Online. 2020. PMID: 32143647 Free PMC article.
References
-
- Guzman RJ, Hirschowitz EA, Brody SL, Crystal RG, Epstein SE, Finkel T. In vivo suppression of injury-induced vascular smooth muscle cell accumulation using adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(22):10732–6. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

