Adverse Effects of Cholinesterase Inhibitors in Dementia, According to the Pharmacovigilance Databases of the United-States and Canada
- PMID: 26642212
- PMCID: PMC4671709
- DOI: 10.1371/journal.pone.0144337
Adverse Effects of Cholinesterase Inhibitors in Dementia, According to the Pharmacovigilance Databases of the United-States and Canada
Abstract
This survey analyzes two national pharmacovigilance databases in order to determine the major adverse reactions observed with the use of cholinesterase inhibitors in dementia. We conducted a statistical analysis of the Food and Drug Administration Adverse Event Reporting System (FAERS) and the Canada Vigilance Adverse Reaction Database (CVARD) concerning the side effects of cholinesterase inhibitors. The statistics calculated for each adverse event were the frequency and the reporting odds ratios (ROR). A total of 9877 and 2247 reports were extracted from the FAERS and CVARD databases, respectively. A disproportionately higher frequency of reports of death as an adverse event for rivastigmine, compared to the other acetylcholinesterase inhibiting drugs, was observed in both the FAERS (ROR = 3.42; CI95% = 2.94-3.98; P<0.0001) and CVARD (ROR = 3.67; CI95% = 1.92-7.00; P = 0.001) databases. While cholinesterase inhibitors remain to be an important therapeutic tool against Alzheimer's disease, the disproportionate prevalence of fatal outcomes with rivastigmine compared with alternatives should be taken into consideration.
Conflict of interest statement
Figures
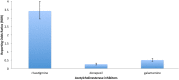
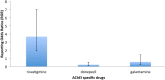
References
-
- Administration USF and D. FDA Adverse Event Reporting System (FAERS) [Internet]. 2014. Available: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveil...
-
- Health Canada. Canada Vigilance Adverse Reaction Online Database [Internet]. 2014. Available: http://www.hc-sc.gc.ca/dhp-mps/medeff/databasdon/index-eng.php
-
- Medicine USNL of. Dementia: MedlinePlus [Internet]. Available: https://www.nlm.nih.gov/medlineplus/dementia.html
-
- World Health Organisation. the Epidemiology and Impact of Dementia Current State of Dementia: Current State and [Internet]. 2015 pp. 1–4. Available: http://www.who.int/mental_health/neurology/dementia/dementia_thematicbri...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

