NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component
- PMID: 26642356
- PMCID: PMC4862588
- DOI: 10.1038/ni.3333
NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component
Abstract
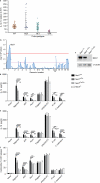
The NLRP3 inflammasome responds to microbes and danger signals by processing and activating proinflammatory cytokines, including interleukin 1β (IL-1β) and IL-18. We found here that activation of the NLRP3 inflammasome was restricted to interphase of the cell cycle by NEK7, a serine-threonine kinase previously linked to mitosis. Activation of the NLRP3 inflammasome required NEK7, which bound to the leucine-rich repeat domain of NLRP3 in a kinase-independent manner downstream of the induction of mitochondrial reactive oxygen species (ROS). This interaction was necessary for the formation of a complex containing NLRP3 and the adaptor ASC, oligomerization of ASC and activation of caspase-1. NEK7 promoted the NLRP3-dependent cellular inflammatory response to intraperitoneal challenge with monosodium urate and the development of experimental autoimmune encephalitis in mice. Our findings suggest that NEK7 serves as a cellular switch that enforces mutual exclusivity of the inflammasome response and cell division.
Figures

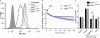


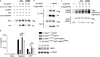

Comment in
-
The NEK-sus of the NLRP3 inflammasome.Nat Immunol. 2016 Mar;17(3):223-4. doi: 10.1038/ni.3391. Nat Immunol. 2016. PMID: 26882252 No abstract available.
References
-
- Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol. Rev. 2011;243:136–151. - PubMed
-
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. - PubMed
-
- Misawa T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14:454–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

