Thermal decomposition of nano-enabled thermoplastics: Possible environmental health and safety implications
- PMID: 26642449
- PMCID: PMC4707061
- DOI: 10.1016/j.jhazmat.2015.11.001
Thermal decomposition of nano-enabled thermoplastics: Possible environmental health and safety implications
Abstract
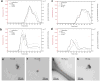
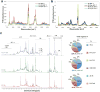
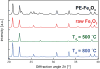
Nano-enabled products (NEPs) are currently part of our life prompting for detailed investigation of potential nano-release across their life-cycle. Particularly interesting is their end-of-life thermal decomposition scenario. Here, we examine the thermal decomposition of widely used NEPs, namely thermoplastic nanocomposites, and assess the properties of the byproducts (released aerosol and residual ash) and possible environmental health and safety implications. We focus on establishing a fundamental understanding on the effect of thermal decomposition parameters, such as polymer matrix, nanofiller properties, decomposition temperature, on the properties of byproducts using a recently-developed lab-based experimental integrated platform. Our results indicate that thermoplastic polymer matrix strongly influences size and morphology of released aerosol, while there was minimal but detectable nano-release, especially when inorganic nanofillers were used. The chemical composition of the released aerosol was found not to be strongly influenced by the presence of nanofiller at least for the low, industry-relevant loadings assessed here. Furthermore, the morphology and composition of residual ash was found to be strongly influenced by the presence of nanofiller. The findings presented here on thermal decomposition/incineration of NEPs raise important questions and concerns regarding the potential fate and transport of released engineered nanomaterials in environmental media and potential environmental health and safety implications.
Keywords: Aerosol; Incineration; Life cycle assessment; Nano-EHS; Nanotechnology.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures
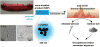

References
-
- Keller AA, McFerran S, Lazareva A, Suh S. J Nanopart Res. 2013;15
-
- BASF. BASF Report 2011: Economic, environmental and social performance. 2011
-
- Stark WJ, Stoessel PR, Wohlleben W, Hafner A. Chem Soc Rev. 2015;44:5793. - PubMed
-
- Wohlleben W, Meier MW, Vogel S, Landsiedel R, Cox G, Hirth S, Tomovic Z. Nanoscale. 2013;5 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

