Prefrontal-hippocampal pathways underlying inhibitory control over memory
- PMID: 26642918
- PMCID: PMC5106245
- DOI: 10.1016/j.nlm.2015.11.008
Prefrontal-hippocampal pathways underlying inhibitory control over memory
Abstract
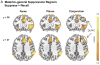
A key function of the prefrontal cortex is to support inhibitory control over behavior. It is widely believed that this function extends to stopping cognitive processes as well. Consistent with this, mounting evidence establishes the role of the right lateral prefrontal cortex in a clear case of cognitive control: retrieval suppression. Retrieval suppression refers to the ability to intentionally stop the retrieval process that arises when a reminder to a memory appears. Functional imaging data indicate that retrieval suppression involves top-down modulation of hippocampal activity by the dorsolateral prefrontal cortex, but the anatomical pathways supporting this inhibitory modulation remain unclear. Here we bridge this gap by integrating key findings about retrieval suppression observed through functional imaging with a detailed consideration of relevant anatomical pathways observed in non-human primates. Focusing selectively on the potential role of the anterior cingulate cortex, we develop two hypotheses about the pathways mediating interactions between lateral prefrontal cortex and the medial temporal lobes during suppression, and their cellular targets: the entorhinal gating hypothesis, and thalamo-hippocampal modulation via the nucleus reuniens. We hypothesize that whereas entorhinal gating is well situated to stop retrieval proactively, thalamo-hippocampal modulation may interrupt an ongoing act of retrieval reactively. Isolating the pathways that underlie retrieval suppression holds the potential to advance our understanding of a range of psychiatric disorders characterized by persistent intrusive thoughts. More broadly, an anatomical account of retrieval suppression would provide a key model system for understanding inhibitory control over cognition.
Keywords: Anterior cingulate; Forgetting; Hippocampus; Inhibitory control; Nucleus reuniens; Retrieval suppression.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Andersen P, Holmqvist B, Voorhoeve PE. Entorhinal activation of dentate granule cells. Acta Physiologica Scandinavica. 1966;66:448–460. - PubMed
-
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nat. 2001;410(6826):131–134. - PubMed
-
- Anderson MC, Huddleston E. Towards a Cognitive and Neurobiological Model of Motivated Forgetting. In: Belli RF, editor. True and false recovered memories: Toward a reconciliation of the debate. Vol. 58: Nebraska Symposium on Motivation. New York: Springer; 2011. - PubMed
-
- Anderson MC, Ochsner K, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover G, Gabrieli JDE. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

